The field of percutaneous coronary intervention (PCI) has rapidly evolved since its inauguration in 1977. Its constant adaptation and advancement have allowed it to remain a mainstay in the treatment of patients with coronary artery disease and acute coronary syndromes. Logically, the majority of technological advancements within the field have focused on ensuring patient safety and expanding the scope of which transcatheter interventions can be used. While there have been significant innovations in what we can do, adaptations in how we perform PCI have lagged behind. As a result, operator fatigue and occupational hazards related to both radiation exposure and orthopedic injury from prolonged standing remain top concerns of interventional cardiologists. Robotic-assisted PCI (R-PCI) is one of the novel innovations within interventional cardiology, aiming to address both occupational hazards for the operator alongside procedural quality and safety improvement for the patient. We review the current role of the robot in the catheterization laboratory, the current systems available for use, and our experience with R-PCI.
The Potential of Robotics in the Catheterization Laboratory
When initially conceptualized, the appeal of a robotic system in the catheterization laboratory reflected that seen in the operating room, where the robot could provide a means of standardizing procedural precision and reproducibility while also addressing occupational hazards of the primary operator. The concept was to create a remote system that would allow for the primary operator to sit behind a shielded console, colloquially termed the ‘cockpit’, thereby reducing radiation exposure and orthopedic injury from prolonged standing at the bedside in a full lead apron. There has been widespread recognition of the risks of long-term radiation exposure, with a definitively increased risk of cataracts and numerous malignancies involving the thyroid, brain, and bone marrow.1–3 Lead shielding has remained the mainstay of radiation protection for staff throughout the decades. While its armamentarium has expanded from lead aprons to include more novel ideas, such as lead-lined gloves and hats, and ‘zero-gravity’ lead (ceiling-suspended lead aprons), the overall paradigm remains unchanged, focusing on personal protective equipment. The incidence of orthopedic injury amongst interventional cardiologists remains alarmingly high, with 49% of interventionalists reporting at least one orthopedic injury in a 2014 nationwide survey.4,5 Robotic-assisted PCI offers a novel modality that can combat both radiation exposure and orthopedic injury for the interventionalist.
The robotic system also provides a potential for precision of device delivery that could supersede what can be achieved by the human eye, particularly when standing a foot away from the screen. The ability to control the robotic arm to move equipment sub-millimeter amounts with such accuracy would certainly be beneficial in the interventional cardiology realm, where accurate stent and balloon placement is critical. Furthermore, the algorithmic capability of a robotic system could create the potential for standardization of the procedure by equalizing the skill set of operators in guidewire navigation and device delivery.
Removing the primary operator from the bedside opens the possibility of remote procedures, a concept that has colloquially been termed ‘telestenting’. The idea of remote operator PCI seemed futuristic a decade ago, but Patel et al. proved its feasibility in early 2019.6 They had a primary operator perform robotic PCI for five patients with type A coronary lesions while situated 20 miles away from the catheterization laboratory. The operator was able to perform the robotic PCI successfully without conversion to a manual approach in all of the patients. In early 2020, Madder et al. demonstrated the feasibility of transcontinental telestenting in preclinical models, where the operator was located over 3,000 miles away from the ex vivo model.7 They found that there was no difference in performance and safety when telestenting was performed regionally versus transcontinental. As would be expected, they found that there was greater latency of media transmission in the transcontinental cases, although this was qualified as imperceptible by the operators. Telestenting is still in its infancy but ultimately could serve a multitude of purposes. Its biggest appeal has been to improve PCI access in remote and underserved regions. It could at the very least allow experts to aid in complex procedures from afar, widening the reach of these highly skilled operators. Furthermore, in the era of the COVID-19 pandemic, we must consider its utility in treating highly infectious patients while keeping our staff safe.
Systems
There are currently two commercially available robotic systems for PCI: the CorPath (Corindus) and the R-One (Robocath). The CorPath 200 was the first PCI robotic system to receive Food and Drug Administration clearance in 2005 and Corindus has since introduced their second-generation system, CorPath GRX. The R-One system by Robocath received a CE marking in February 2019 and is currently available throughout Europe and Africa. It has similar features to the first iteration of the Corindus Vascular System, the CorPath 200. While it currently lags in technical advancements, the inherent rivalry between systems is encouraging to promote innovation within the field.
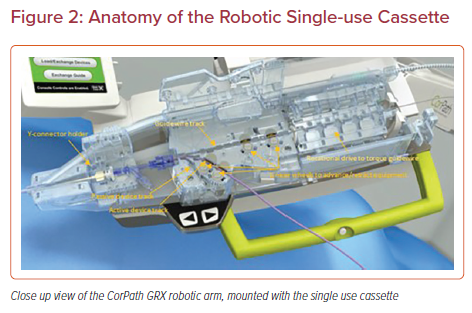
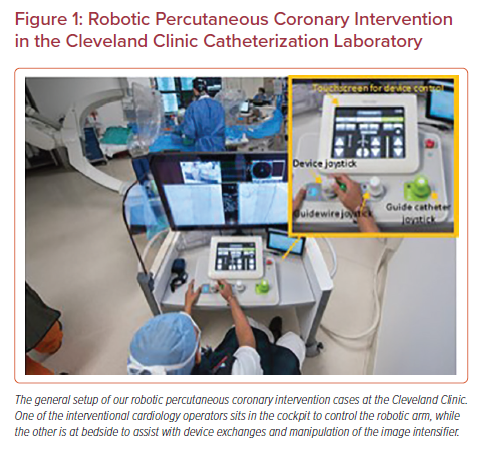
The systems are made up of two subunits – the robotic arm that is stationed bedside and the interventional cockpit, where the primary operator will sit to perform the PCI (Figures 1 and 2). The robotic arm is stationed at the caudal end of the table and can be retracted to the side when not in use. A single use cassette is attached to the arm in a sterile fashion and contains three equipment tracks: one designated for the guidewire, an active track for device advancement using the robot, and a passive track for devices that are left in place (such as a guide catheter extension) or that are used in a hybrid approach with manual advancement of the device by the bedside operator.
The interventional cockpit can be placed in the catheterization laboratory behind a radiation shield or alternatively can be placed in the control room. Either way, the primary operator is able to perform the PCI without the orthopedic burden of a lead apron. The setup of the cockpit varies amongst the different systems, but the general concept is similar. There is a high-resolution screen that allows the operator to monitor the fluoroscopy screen, hemodynamics, and the bedside operations simultaneously (the latter from a camera that is setup above the table). The gears of the robot are then controlled by individual joysticks, which manipulate the equipment in an assigned track. Both systems have a joystick for the guidewire and one for the device, while the CorPath GRX has a third joystick for control of the guide catheter.
Data
A number of observational studies have been published evaluating the feasibility, safety, and potential benefits of R-PCI for both patients and operators. Given the relatively recent approval of the Robocath R-One for commercial use, the majority of data have come from use of the Corindus CorPath robot models. The preclinical randomized trial for the R-One device was conducted by three operators in 2017, with a 100% technical success rate and no major adverse cardiac events.8 The data overall have been positive in terms of safety, feasibility, and short-term outcomes. A recent meta-analysis reflects this, including 148 R-PCI patients from five studies in comparison to 493 patients who underwent manual PCI. They found that operators had lower radiation exposure in the R-PCI group without compromise in total stents per case, fluoroscopy time, or procedural success rates.9
The PRECISE study was the inaugural study to evaluate safety and feasibility of robotic PCI, published by Weisz et al. in 2013.10 This non-randomized, multicenter study enrolled patients with at least 50% coronary stenosis that could be treated with a single stent, with the majority of lesions being classified as type A (28.7%) or B1 (39.6%). These stringent inclusion criteria essentially allowed this study to serve as a proof of concept for R-PCI. The results were favorable, with technical success achieved in 162 of 164 patients (98.8%) without conversion to manual operation. At 30-day follow-up, there were no deaths, strokes, non-fatal MI, or target lesion revascularization. Perhaps the most exciting result though was the significant reduction in median radiation exposure of the primary operator, which was found to be 95.2% less during time spent in the interventional cockpit compared to time spent at the traditional table position.
CORA-PCI was designed to assess the feasibility of R-PCI for more complex patient lesions, whereby the inherently longer procedural duration would make the robot an appealing tool.11 This was a non-randomized single center comparison of patients undergoing R-PCI – notably performed by a single operator – versus those undergoing manual PCI in the CathPCI registry. Type C lesions made up the majority of those compared in both groups of the study. The authors found that the total procedure time was slightly longer in the R-PCI group (43 minutes versus 34 minutes; p=0.007), but there were no differences in contrast volume or dose area product of the patient between the two groups. Within the R-PCI group, 81.5% of cases were completed entirely robotically while 7.4% required conversion to manual approach due to technical limitations of the robotic platform or limited guidewire or guide catheter support (this was notably using the CorPath 200, which does not have robotic guide catheter manipulation). There were three patients in the robotic cohort who required at least partial manual assistance due to an adverse event. Two of these were due to coronary dissection following balloon predilation and one was due to acute vessel closure during advancement of a guidewire. All three cases were successfully treated with manual stent placement and without further issue. These findings overall supported the feasibility of R-PCI for more complex lesions. This was further supported by a recent publication by Hirai et al., who published data from two centers that retrospectively compared 49 patients who underwent chronic total occlusion (CTO) PCI via R-PCI versus 46 patients who were treated with traditional PCI.12 R-PCI for CTOs was not associated with excess adverse events with significant reduction in radiation exposure for the primary operator, with on average ~48% of time spent in the cockpit.
While data from these early studies were favorable in terms of benefit for the operator without harm to the patients, there was no clear evidence of a benefit to the patients until recently. Patel et al. published data from a single center observational study comparing outcomes of traditional PCI versus robotic PCI.13 A total of 310 patients (31.1%) were included in the R-PCI group and 686 patients (68.9%) in the traditional PCI group. Twenty-two patients in the R-PCI group required conversion to a manual approach, but were included in the R-PCI group for an intention-to-treat analysis. After propensity score matching, they found that R-PCI was actually associated with a significant reduction in radiation exposure to the patient (mean dose area product 4,734 cGycm2 [range: 2,695–7,746] versus 5,746 cGycm2 [3,751–7,833]; p=0.003). With the interventionalist in the cockpit, table height was not limited by the operator’s ergonomic comfort and so it could be raised to minimize radiation exposure to the patient. There was no significant difference in fluoroscopy time or contrast volume between the two groups, though total procedural time was notably higher within the R-PCI group, presumably related to time required to set up the robot (mean: 27 minutes [range: 21–40] versus mean: 37 minutes [range: 27–50]; p<0.0005).
Robotic-assisted Percutaneous Coronary Intervention in the Real World: Our Experience
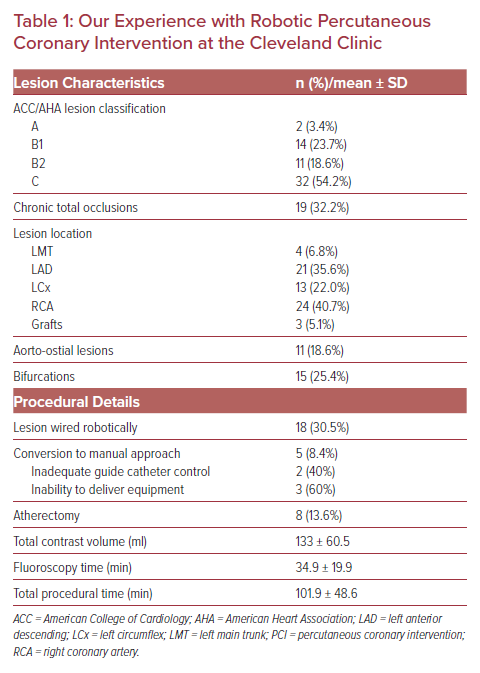
Our institution performed our first robotic-assisted PCI in August 2019 using the CorPath GRX Vascular Robotic System by Corindus. Since then, we have performed just over 100 R-PCI cases, comprising everything from left main trunk stenting to CTO lesions and single-access Impella cases (Table 1).14 As an academic institute, our interventional cardiology fellows are involved in all PCI cases, including robotic ones. For R-PCI, the interventional cardiology fellow will spend their first few cases at the bedside learning how to operate the robotic arm and then will have the opportunity to reside in the cockpit during interventions, while the attending physician acts as the bedside operator. Our cockpit is lead-lined itself and situated within the lab, allowing for direct communication between both operators. We have found that there is a relatively small learning curve for use of the robot, with the biggest challenge being converting from tactile and visual feedback to only visual cues.
The robotic arm is attached to the end of the bed and is then brought into position and draped in a sterile fashion when needed. It takes less than 5 minutes to drape and position the robot at the beginning of the procedure. Each device exchange is then performed by the bedside operator and will usually take less than a minute to perform, whereby the previous device is removed and the next one loaded and advanced to the 90/100 cm mark, depending on guide catheter length. In our experience, there is a marginal increase in procedural time with a robotic approach, although not necessarily fluoroscopic time.
Understanding the current limitations of the robot in addition to its potential has allowed us to select appropriate cases for a robotic approach (Table 2). One of the major limitations of the available iterations is a lack of adequate guide catheter control. All of the current robotic systems require the operator to manually obtain vascular access and engage the coronary artery with the guide catheter. Only after the guide catheter has been engaged should it be connected to the robotic arm. The guide catheter is locked into place within the cassette, which inhibits further manual adjustments unless the robotic arm is disconnected. The CorPath GRX system has an upgraded feature in attempt to allow for robotic guide catheter control through joystick manipulation. This is certainly a vast improvement from the prior iteration, there was no alternative to disconnecting the system and reengaging the coronary manually. While ideal for subtle adjustments of the guide catheter during the case, we have found it very difficult to reengage the guide catheter entirely using the robot alone despite anecdotal reports of this being possible. Instead, we find it much more efficient to briefly disconnect the guide catheter for manual manipulation. The joystick allows for advancement and retraction as well as torquing of the guide catheter. It should be noted that advancement and retraction will result in movement of all connected devices, and so guidewires and catheters should be watched closely to prevent inadvertent complications. We have also learned that 90 cm guide catheters, often used in CTO cases for retrograde access, are often too short to complete PCI robotically. When connected to the robotic arm, these shorter guide catheters create too much tension to maintain stable coronary engagement. In taller patients, 90 cm guide catheters lack the necessary length to connect to the robotic cassette. With the first generation of the CorPath robotic system, aorto-ostial lesions were difficult due to the inability to control the guide catheter. Now with the upgraded system, ostial lesions are feasible as long as the operator has adequate ability to actively control the guide catheter. If the patient has tortuous anatomy or radial spasm, an alternative approach should be considered.
Similar to guide catheter manipulation with the robot, robotic guidewire navigation requires the operator to rely only on visual feedback. The robot can accommodate any 0.014 inch guidewire within its track. It should be noted that the current robotic iterations do not support the use of pressure guidewires for invasive hemodynamics, though the guidewire can be used once disconnected from the modular connector. One of the major appeals of R-PCI is the computational capability of the robot, which could allow for the creation of algorithms based on techniques of advanced operators – essentially leveling the playing field for all interventionalists. Corindus recently released its upgraded software for the CorPath GRX, including a proprietary program called ‘Rotate on Retract’, which is the first installation of its proprietary technIQ Smart Procedural Automation. The automation was designed to improve procedural reproducibility independent of an operator’s individual skills by creating algorithms based on the experience of highly skilled interventional cardiologists. When the ‘Rotate on Retract’ feature is activated, the robot will automatically rotate the guidewire 270o with each retraction input, so as to set the operator up for an alternative approach as one would at the bedside. Objective, preclinical data were favorable when the feature was used for wiring coronaries in a porcine model.15 It is also possible to torque the guidewire manually using the joystick, although there is a significant delay between input and wire response. We look forward to the implementation of future automated movements including spin, wiggle, Dotter, and constant speed, all of which could greatly improve robotic navigation and lesion crossing.
When considering device delivery, the robot is impartial in terms of device companies and can accommodate rapid exchange catheters up to 7 Fr (although off label, we have been able to use 8 Fr guide catheters without issue). Over-the-wire equipment, such as rotational or orbital atherectomy systems, cannot yet be accommodated for R-PCI. While it is possible to perform atherectomy and then transition to a robotic approach, this cannot be done with a hybrid approach. Microcatheters, a fundamental tool for PCI of chronic total occlusions, cannot be accommodated either, which makes attempts at robotic wiring of these lesions implausible with the current systems. The gears within the system also prohibit the use of fragile catheters that lack a rigid hypotube, such as the Beta-Cath for brachytherapy (Novoste) or the Dragonfly Optis catheter for optical coherence tomography imaging (Abbott).
Intravascular imaging is a crucial step in our daily PCI practice so it is imperative that we can incorporate it into the workflow of R-PCI. The robotic systems cannot accommodate automatic pullback runs of rotational devices, although this is not an issue in our laboratory as we predominantly use solid-state intravascular ultrasound (IVUS) imaging. While it is possible to perform a pullback robotically using the device joystick, we have found that the robotic gears can occasionally damage the IVUS catheter and render it defective. This often occurs in calcified or tortuous lesions, where the robotic gears slip as the IVUS catheter is gripped within the vessel. As a large majority of our patients have at least one of these two vessel characteristics, we prefer to use IVUS in a hybrid approach by placing the catheter in the passive device track, which allows the catheter to be advanced and retracted manually by the bedside operator.
One of the major benefits that we have found in using the R-PCI relates to its ability to assist in precision with measurements. The CorPath GRX has patented software that allows for lesion length measurement during pullback of any intracoronary device. The distal marker or end of the device is placed at the distal target lesion border and the device position counter is then reset to zero. The operator can then retract the device until the distal marker is at the proximal target lesion border, where the total length traveled will be displayed on screen in mm. Appropriate stent length, even when multiple stents are needed, can then be chosen without any guesswork. It should be noted that accuracy of the software depends on 1:1 joystick input to movement responsiveness of the device, which is not always the case. If the device is stuck, the program will still count and render an inaccurate measurement. We find that this often happens with the IVUS catheter but is less of an issue with balloons or stents. Prior data have also suggested that R-PCI lowers the incidence of longitudinal geographic miss compared to manual PCI.16 This is likely related to a combination of improved measurement accuracy with the robotic software, improved visualization given closer screen proximity for the operator, and finally the controlled pinning of the device during inflation and deployment by the robot gears.
While we have found it to be relatively uncommon to need to abort a robotic approach, conversion to manual PCI is quite straightforward. The guide catheter must be unlocked from the robotic arm, which thereby frees up the catheter and equipment for manual use and the robot can then be positioned at the end of the bed. This step is not particularly cumbersome but does add about a minute of procedural time. On our experience, we have not found this to be detrimental to our workflow.
Overall, we have found that R-PCI can be easily incorporated into daily use for non-complex coronary lesions and is certainly feasible for complex coronary lesions. Catheterization laboratories do have to be thoughtful about resource utilization and financial burden of novel equipment. Encouragingly, data are starting to emerge suggesting that the cost of R-PCI is comparable to manual cases overall, with the expected increase in direct supplies cost related to the single-use robotic equipment.16,17 At our institution, we see this cost can be seen as a long-term investment for our operators’ radiation safety and long-term health with reduced orthopedic burden from standing in lead. Economically, R-PCI has the potential to improve our accuracy leading to cost savings in terms of per-case stent use; preliminary data have suggested that this holds true, though dedicated studies are needed to validate this potential.17
Conclusion
There is no doubt that R-PCI has significant potential for expanding our capabilities in the catheterization laboratory, whether regarding standardization of the procedure, improved operator endurance, or the prospect of telestenting. While we have made significant strides in the technical capabilities of R-PCI over the past decade, robotic systems will need to continue to evolve to adapt for the needs of the procedure. With the influx of data supporting intravascular imaging for optimization of PCI, it is imperative that upgrades be made so that these devices can seamlessly and reliably be used within the robotic system. Likewise, the routine need for over-the-wire equipment in complex PCI cases must be addressed. While there is still a way to go, it is important to recognize how far we have come. We must continue to purposefully practice the workflow of R-PCI to improve our efficiency and unleash the potential of this technology.