In 1939, Harrison introduced cardiogenic shock (CS) as a specific entity and differentiated it from other forms of shock.1 Acute MI (AMI) is the most common cause of CS and has a mortality rate of up to 50%, which has changed little in the past two decades.2
In the early years of interventional AMI therapy, intracoronary thrombolytic agents were used to dissolve thrombi.3,4 Later, in 1977, Gruentzig performed the first percutaneous coronary artery balloon angioplasty. Nearly one decade later in 1986, the first bare metal stent was implanted. Since then, coronary artery stents have undergone significant development, spawning generations of drug-eluting stents.5 In 1999, Hochman et al. published one of the first major randomized controlled trials (RCTs) in the field, the SHOCK trial, proving early revascularization in AMI-associated CS (AMICS) to be the cornerstone of successful treatment and reduction in mortality for these patients.6
Another fundamental pillar for supporting AMICS patients is to bridge hemodynamic instability with mechanical circulatory support (MCS) devices. More than 50 years after the intra-aortic balloon pump (IABP) was developed in 1968 as the first MCS technology, the arsenal has increased considerably. Currently, IABP, the Impella (a miniaturized ventricular assist device; Abiomed) and extracorporeal life support (ECLS) circuits are the most common devices for acute and short-term MCS in CS.
Technical advances in the performance and manageability of MCS devices have led to their widespread availability and more frequent use. However, despite the remarkable increase in short-term MCS use, there is still little evidence from RCTs showing any significant improvement in strong outcome parameters.
Landmark Trials and a Slow Evolution
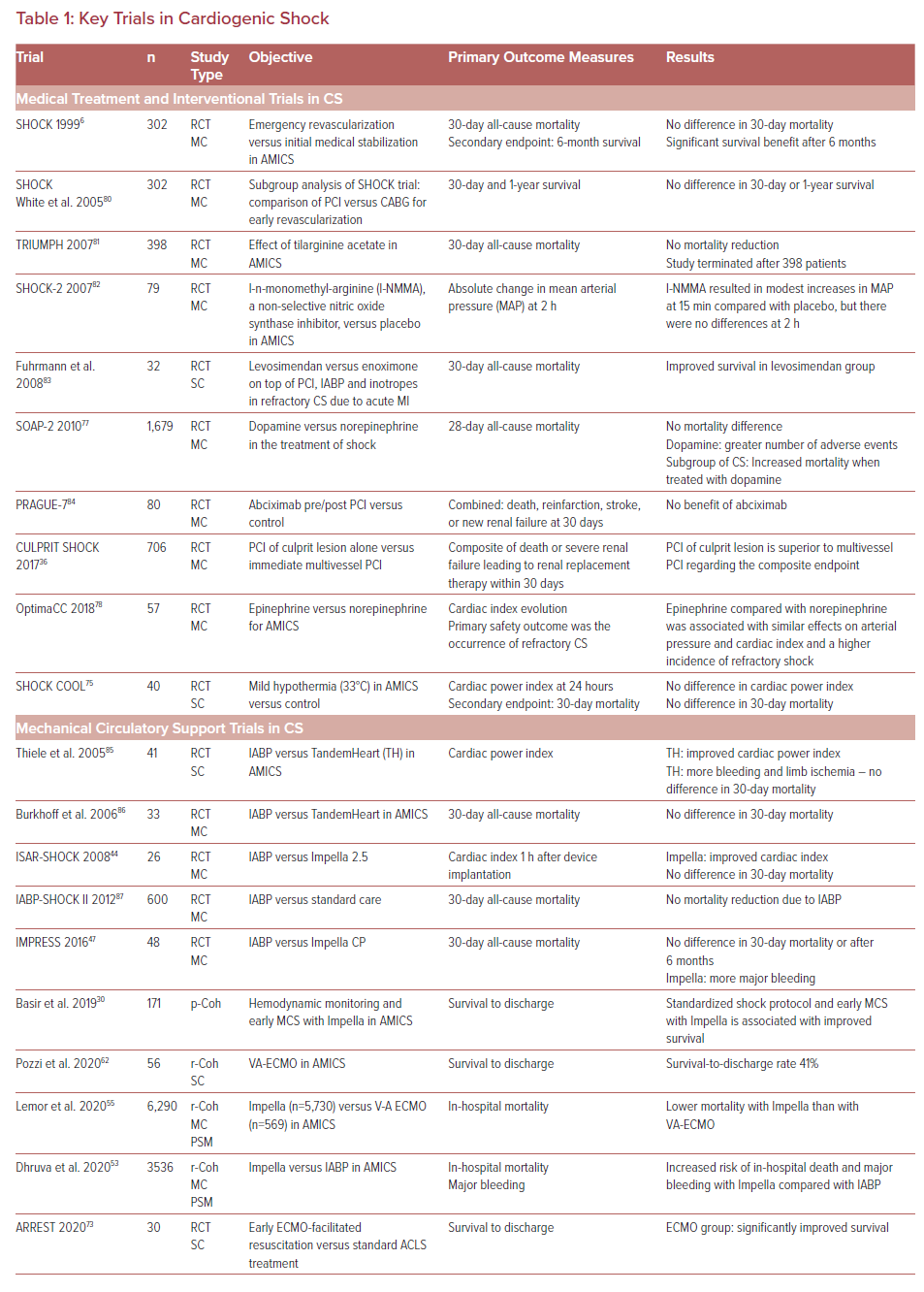
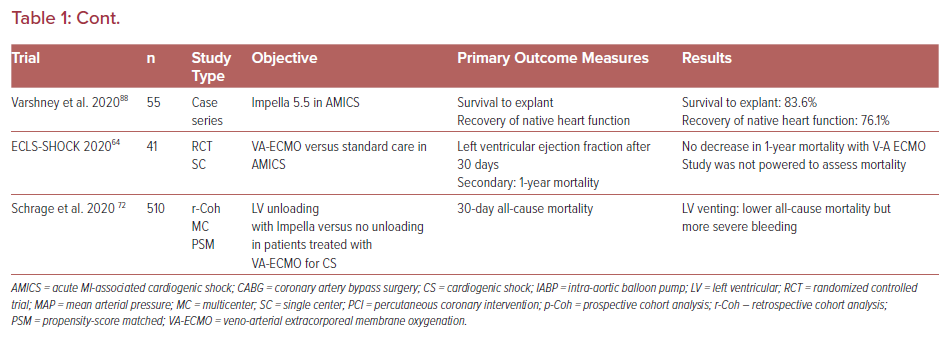
Table 1 provides an overview of the key trials in medical and mechanical therapy of CS in the past 20 years. The initial trials of MCS were predominantly retrospective cohort analyses with all their known limitations. Since the first use of a heart-lung machine by Gibbon in 1965, meaningful scientific interrogation of MCS modalities has evolved slowly.
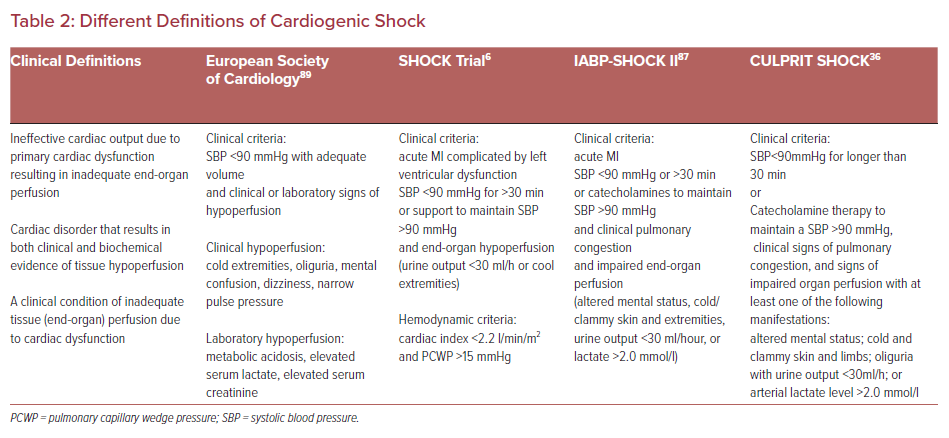
First, a major issue in performing and comparing trials in the field of CS and MCS related to CS is the absence of a standard definition. Table 2 summarizes different definitions of CS by the US and European societies as well as those of some landmark trials. In addition, differing primary endpoints among study groups further complicate the situation.
Second, although AMI is the most common cause of CS, there are many more phenotypes of patients with acute heart failure, which adds complexity to screening and randomizing patients for a trial in time-pressured circumstances.2 Defining a specific study population can be problematic and obtaining patients’ informed consent difficult. Furthermore, blinding is normally not possible.
Consequently, the first randomized controlled trials (RCTs) in the field of MCS were small trials enrolling fewer than 50 patients. Table 1 provides an overview of the RCTs in MCS. Most of the trials were aborted because of low recruitment rates, highlighting one of the major problems in prospective RCTs in MCS.7 Other than increasing the number of recruiting centers in multicenter trials, the hub and spoke model proposed by van Diepen et al. could improve recruitment yield.8
Furthermore, there are ethical issues around randomizing critically ill patients to be supported with MCS, which is often thought to be a last-chance treatment option, and such issues will require careful consideration when designing a trial. In this context, the study protocol of the DAWN trial, explained by Samsky et al., could be helpful.9,10
Moreover, the necessary infrastructure participating sites need to establish to successfully undertake trials in MCS is complex, costly, and can be delivered by only a limited number of select centers.11
Finally, volume/outcome relationships for MCS programs are increasingly well documented, and the need for dedicated cardiogenic shock centers has become apparent.12,13
Current Topics and Updates from Recent Studies
Definition and Diagnosis of Cardiogenic Shock
Therapeutic intervention is often time critical, not least to minimize secondary end-organ damage, so early diagnosis is key.
Recently, Chioncel et al. again highlighted the significance of early detection of tissue hypoperfusion in patients with evolving or established CS.14 Shortly afterwards, Chioncel et al. published a position statement on behalf of the European Society of Cardiology Heart Failure Association, presenting a new definition of CS that focuses on the importance of hypoperfusion and organ dysfunction.15 In this context, hypotension is no longer a required criterion, and the definition now includes normotensive CS.
Baran et al. presented the Society for Cardiovascular Angiography and Interventions (SCAI) clinical expert consensus statement on the classification of cardiogenic shock, defining a grading system of CS ranging from stage A with patients At risk, through to B and C (Beginning and Classic CS) – then to D and E (Deteriorating patients and those in Extremis).16 This classification was shown to correlate with both in-hospital mortality and mortality after hospital discharge.17–20
Ceglarek et al. published the results of a CULPRIT-SHOCK biomarker sub-study and presented a novel, fast, and objective, biomarker-based mortality risk score for patients with AMICS.21 Based on an evaluation of cystatin c, lactate, interleukin-6 and N-terminal pro-B-type natriuretic peptide, they established and validated the CLIP score as a mortality risk predictor.
Hemodynamic Monitoring
Cardiac output (CO) or cardiac index (CI) are key parameters in evaluating patients with CS. There is an ongoing debate over the role of the pulmonary catheter (PAC). Although the PAC was first introduced and studied in the mid-1970s, 50 years later there is still no clear evidence concerning its significance.22
The use of PACs has decreased over the years, probably influenced by the ESCAPE trial and other studies which showed there was no benefit from PAC monitoring.23,24 However, this study did not enroll CS patients. To date, PACs have never been studied specifically in a CS population. Existing data were obtained from different study populations and reveal contradictory results.25 In a propensity score-matched retrospective cohort study with the remarkable number of 9,431,944 patients, Hernandez et al. showed that the use of PACs in patients with heart failure was, indeed, associated with increased mortality (9.9% versus 3.3%; OR 3.96; p<0.001); however, in patients with CS, PACs were associated with lower mortality (35.1% versus 39.2%; OR 0.91; p< 0.001).26 Another recently published study and two expert consensus articles support the use of PACs in CS.27–29 Consequently, CS treatment algorithms in current studies include PAC monitoring.12,30
However, because of concerns about the safety of PACs, alternatives, such as transpulmonary thermodilution (TPTD), are being investigated. Only a few studies have addressed the accuracy and utility of TPTD methods in patients with CS. Schmid et al. compared values derived from TPTD and PAC in a population of 11 patients with CS and found they gave identical results.31 Zhang et al. prospectively randomized 60 patients with AMICS to a pulse contour (invasive) continuous cardiac output (PiCCO)-guided therapy group versus standard care and demonstrated a mortality benefit in patients treated with PiCCO, a TPTD method.32
Technical advances in the past decade have opened up new avenues of noninvasive cardiac output monitoring (NICOM). While setting out to assess these innovations, the recently published NICOM study reported disappointing results, showing one NICOM modality to be unreliable in measuring cardiac output in patients with decompensated heart failure and CS.33
Reperfusion strategies
Early revascularization has been accepted for many years as the key intervention to treat patients with AMI to prevent deterioration to CS and is firmly established in current guidelines and recommendations.6,34,35 A significant number of patients with acute MI, however, present with more than one coronary lesion. The CULPRIT-SHOCK trial prospectively showed a culprit-lesion-only percutaneous coronary intervention (PCI) strategy to be superior to an immediate multivessel PCI in patients presenting with AMICS.36
Another aspect was identified in a sub-study of the CULPRIT-SHOCK trial. Guedeney et al. were able to show that transradial artery access compared with transfemoral artery access was associated with a lower 30-day death rate (34.7% versus 49.7%; adjusted OR 0.56; 95% CI [0.33–0.96]) and a lower rate of renal replacement therapy requirement (5.9% versus 15.9%; adjusted OR 0.40; 95% CI [0.16-0.97]).37 However, this benefit could not be confirmed at the 1-year follow up. The causes for the improved short-term endpoints warrant further research.
Mechanical Circulatory Support
Intra-aortic Balloon Pump
In 2013, Thiele et al. published the IABP-SHOCK II trial, the first adequately powered prospective, randomized multicenter trial comparing IABP against controls in a CS population, which showed no improvement in 30-day, 12-month, or 6-year mortality rates.38,39 Consequently, European guidelines do not recommend routine IABP implantation in CS (class 3b).40 The last US guideline for managing AMICS from 2013 did not yet include the IABP-SHOCK II data and issued a class 2a recommendation.35 Later updates, however, downgraded the recommendation for the routine use of IABP.28, 41
In a subgroup analysis of the IABP-SHOCK II trial, Fuernau et al. investigated the impact of timing of IABP on mortality in CS and found no difference whether IABP was implanted before or after PCI.42
Impella
Although the Impella has been shown to provide superior hemodynamic support to IABP, there are conflicting results with respect to hard outcome parameters.43–45 However, these conflicting results originate mostly from studies comparing Impella to other MCS strategies.44,46-48
To date there is only one study, a retrospective, single-center cohort analysis, comparing Impella to medical treatment in patients after out-of-hospital cardiac arrest due to AMI who subsequently present with CS.49 This analysis suggests an Impella-associated survival benefit at hospital discharge and after 6 months.
Scherer et al. recently presented propensity-score matched data from a retrospective analysis comparing Impella CP (n=70) with non-MCS-treated CS patients (n=70).50 While, naturally, there were more bleeding complications in the Impella groups, mortality rates did not differ.
The ISAR-SHOCK trial compared Impella 2.5 (n=12) with IABP (n=13) in patients presenting with AMICS, and revealed a higher CI in the Impella group.44 Mortality, however, was not influenced.
Manzo-Silberman et al. retrospectively compared Impella 2.5 with IABP in 78 patients and found no difference in mortality, but a higher rate of bleeding complications in the Impella group.46 Patients in the Impella group were on higher catecholamine doses (epinephrine 2.3 mg/h versus 1.0 mg/h; p=0.04) and their left ventricular (LV) ejection fraction was lower (25% versus 35%; p=0.01), indicating the groups were not balanced with respect to illness severity.
In 2017, Ouweneel et al. presented the IMPRESS study, the first prospective multicenter trial comparing Impella CP (n=24) with IABP (n=24).47 This study, again, showed no significant difference in mortality rate (50% versus 46%; p=0.92); differences in bleeding complications also failed to reach statistical significance. Notably, all patients in the Impella group and 83% of the IABP group underwent cardiopulmonary resuscitation before device implantation.
A study by Pieri et al., though retrospective, single-centered and non-randomized, also identified aspects warranting further evaluation.48 As in the study by Manzo-Silberman et al., patients in the Impella group (2.5 and CP models) were more critically ill, as indicated by more frequent catecholamine support (93% versus 57%; p=0.002), and were on higher doses of inotropes (indicated by an inotropic score of 8 versus 5; p=0.02). Nevertheless, the 30-day mortality rate tended to be lower in the Impella group (79% versus 94%; p=0.11). In any case, mortality rates of 79% or 94% appear conspicuously high. At 6-month follow-up, LV ejection fraction and cardiac recovery rate were higher in the Impella group. A retrospective comparison of historical cohorts on Impella versus IABP support by Alushi et al. yielded similar results.51
Another remarkable study, by Schrage et al., compared Impella 2.5 and CP with a historical control group from the IABP-SHOCK II trial treated with IABP or medical treatment; it showed no survival benefit for the Impella group, but more bleeding and peripheral vascular complications.52 Adjusting the control group to IABP patients alone did not change the results; however, a comparative analysis after adjusting for medical treatment alone was not performed.
The recently published propensity-matched, registry-based, retrospective cohort study by Dhruva et al. attracted attention after reporting a higher adjusted risk of in-hospital death or major bleeding complications under Impella support compared to IABP.53 This study, however, has been heavily criticized for statistical limitations and incomplete conditions for comparison.54
In contrast, Lemor et al. retrospectively analyzed data of 6,290 patients from the US National Inpatient Sample register to compare Impella with veno-arterial extracorporeal membrane oxygenation (VA-ECMO) in patients with AMICS.55 In this propensity score-matched study, patients treated with Impella had a significantly lower in-hospital mortality rates than those receiving V-A ECMO (26.7% versus 43.3%; OR: 2.10; p=0.021). However, these data are based on the International Classification of Diseases (ICD) and CS was thus identified on the basis of the ICD code, not on hemodynamic parameters. Furthermore, there was no discrimination as to the specific devices or cannulation strategies. Despite these substantial limitations, the impressive number of patients must be acknowledged, and the results are consistent with current findings that VA-ECMO is not, at least not in isolation, the panacea for CS.
On the basis of available data, there are growing calls for limiting the unrestricted use of Impella in AMICS.56 The limitations of these data, however, bring us back to the root of the problem. While propensity-score matched analyses mitigate numerous limiting factors of retrospective studies, they are still not RCTs and significant limitations remain. Well-powered, prospective, multicenter RCTs producing high-quality data to delineate the significance of Impella are still lacking, and numerous other questions, such as those concerning timing of implantation, choice of Impella device, combination with other devices or just suitable patient selection, have not yet been sufficiently addressed.
For example, Nersesian et al. identified a lactate level >8 mmol/l or having received cardiopulmonary resuscitation before implantation as predictors for increased 30-day mortality in a mixed etiology cohort of patients with CS treated with Impella 5 or 5.5.57 Indeed, the use of Impella 5 or 5.5 in CS after cardiopulmonary resuscitation was associated with an increase in 30-day mortality (92% versus 41%, p=0.001).
Another approach, intended to reduce bleeding and other complications, is the ECMELLA 2.0 concept, where single arterial access is used for VA-ECLS and Impella.58 In this context, the results from the currently recruiting DanGer Shock trial are eagerly awaited.59 In this multicenter RCT, Udesen et al. are prospectively comparing Impella CP versus conventional therapy in patients with AMICS.
The recently published consensus statement by the European Association of Percutaneous Cardiovascular Interventions and the Association for Acute Cardiovascular Care recommends Impella CP to be considered for short-term MCS in CS stage C and D with a potentially reversible underlying cause, as a bridge to transplant, or in ventricular assist device candidates.60
Veno-arterial Extracorporeal Life Support
The Extracorporeal Life Support Organization published a position paper in 2019 addressing the nomenclature of ECLS.61 According to this, ECLS is defined as a set of therapies that focus on oxygenation, carbon dioxide removal, cardiac support, or a combination thereof. ECMO is one ECLS entity used for temporary support of patients with respiratory and/or cardiac failure. Therefore, in general, the term ECLS is used in this article; when reviewing former studies that used the term ECMO, this terminology was maintained.
Even though VA-ECLS is significantly older than Impella, high-quality data are even more scarce. The first RCTs that systematically addressed the role of VA-ECLS in CS have only been published in the past 2 years.
The retrospective cohort analyses by Lemor et al. discussed above, which has significant limitations, showed VA-ECLS to be inferior to Impella in treating CS.55
Pozzi et al. recently published a retrospective observational analysis from their institutional database of patients treated with VA-ECMO in AMICS.62 Between 2007 and 2017, they treated 56 patients with VA-ECMO and demonstrated a survival-to-discharge rate of 41.1% (n=23). Notably, the results of a subgroup analysis showed that patients aged ≤60 years had a better chance of survival. This matches the findings of Muller et al., who identified an age of ≥60 years as an independent risk factor for death during an ICU stay.63 However, in Pozzi et al.’s study, the survival rate with VA-ECMO did not substantially exceed common survival rates of CS treated conventionally. Relatively little is known about their local MCS protocols, and it is questionable whether a median of less than six patients per year can support a complex intervention like ECLS to become standard care.
The only published randomized trial comparing VA-ECMO treatment of CS with standard care is the ECLS-SHOCK trial.64,65 Forty-one patients with AMICS were randomized to receive VA-ECMO or not. There was no difference in the primary endpoint of LV recovery. All-cause mortality after 1 year showed no difference either but a trend of lower mortality in the VA-ECLS group was observed (19% versus 38%; p=0.31). Mortality was a secondary endpoint though, and the study was underpowered to detect a difference.
Left Ventricular Unloading and ECMELLA
Despite the ability of VA-ECLS to support cardiac and pulmonary function, there are considerable limitations and disadvantages to this approach. The increase in LV afterload and consecutive rise in LV wall stress impeding recovery has been known about for many years.66–68
The mortality-reducing effect of LV unloading regardless of the method applied (IABP, Impella, right upper pulmonary vein drainage, or transseptal left atrial cannula) has been underlined by recent meta-analyses.69-71
The latest study addressing the combination of VA-ECLS with an Impella for LV-unloading, called ECMELLA, was recently published by Schrage et al.72 This retrospective, international, multicenter, 1:1 propensity-score matched cohort analysis compared 510 patients with CS treated with VA-ECMO with or without LV unloading by Impella. LV unloading was associated with a lower 30-day mortality (HR 0.79; 95% CI [0.63–0.98]; p=0.03). This reduction in mortality was seen even though patients with LV unloading were more likely to experience complications such as severe bleeding (38.4% versus 17.9%; p<0.01), access site-related ischemia (21.6% versus 12.3%; p<0.01), abdominal compartment syndrome (9.4% versus 3.7%; p=0.02), and a requirement for renal replacement therapy (58.5% versus 39.1%; p<0.01). Even though the data give a signal for the beneficial effect of LV unloading, this concept, again, is based on retrospective analysis of observational studies and adequate RCTs are missing.
Extracorporeal Life Support in Cardiopulmonary Resuscitation
Since VA-ECLS is quite easily and rapidly implanted at the bedside, provides biventricular and pulmonary support, and is furthermore of comparatively low cost, it distinguishes itself as a firstline MCS for patients in cardiac arrest.
In 2020, Yannopoulos et al. presented the ARREST trial, comparing ECMO-facilitated resuscitation with standard advanced cardiac life support treatment in patients with out-of-hospital cardiac arrest and refractory VF.73 For just short of a year, they randomly assigned 15 patients to each group, and there was a 36 percentage point better survival rate in the ECMO-facilitated resuscitation group (43% versus 7% survival; HR 0.16; 95% CI [0.06 – 0.41]; p<0.0001). Notably, all survivors in the ECMO group had good cerebral performance scores at 6 months. Although the trial was planned to enroll 77 patients, it was discontinued after the first interim analysis because of the superiority of VA-ECMO.
Hypothermia
The discussion whether mild hypothermia in patients with AMICS but not specifically after cardiac arrest improves morbidity and mortality has been ongoing for several years.74
The SHOCK-COOL trial investigated the impact of therapeutic hypothermia (33°C) for 24 hours in AMICS patients without a history of cardiac arrest.75 The primary endpoint was the cardiac power index after 24 hours; secondary endpoints were several hemodynamic parameters and lactate levels. There was no difference in the cardiac power index or hemodynamic parameters. Lactate levels were higher in the hypothermia group; there were no significant differences in 30-day mortality (60% versus 50%; HR 1.27; 95% CI [0.55–2.94]; p=0.55).
The HYPO-ECMO trial, a prospective, multicenter RCT examining the impact of moderate hypothermia (33–34°C) during VA-ECLS in CS patients, was recently completed and is expected to be published in 2022.76
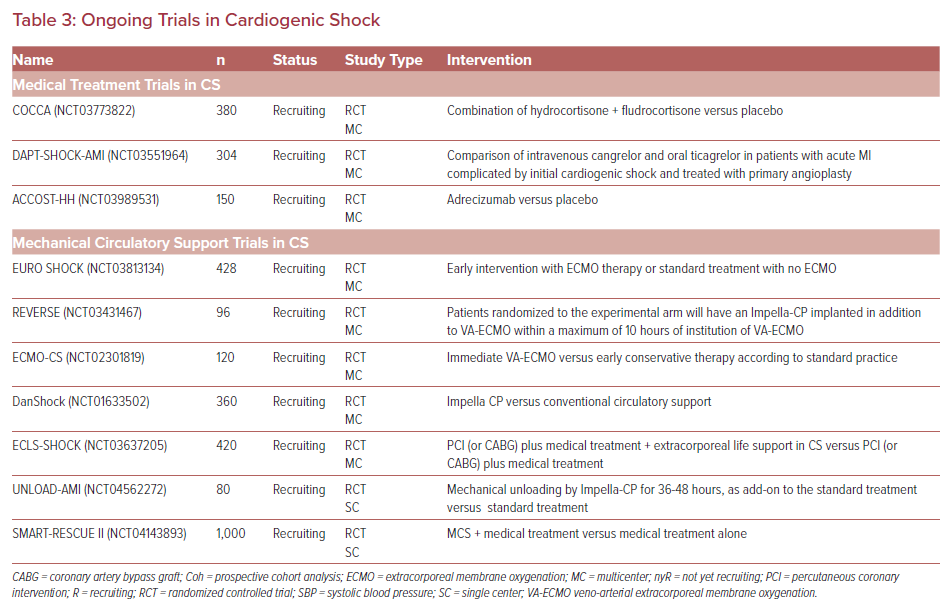
Current Clinical Trials
Table 3 and Supplementary Material Table 1 provide an overview of ongoing clinical trials in the field of CS. Besides some trials investigating medical therapies, a considerable number of MCS studies have been initiated.
Interestingly, despite the conflicting evidence regarding the significance of Impella in CS, only the DanGer trial and the UNLOAD-AMI trial (NCT04562272) are focusing on this question.59 The ECMO-CS trial (NCT02301819) and the EURO-SHOCK trial (NCT03813134) will examine the impact of early ECLS intervention in patients with CS. Eagerly anticipated are the results of the REVERSE trial (NCT03431467) by Schrage et al., who are prospectively investigating the potential superiority of the ECMELLA concept compared with VA-ECLS alone.72
Conclusion
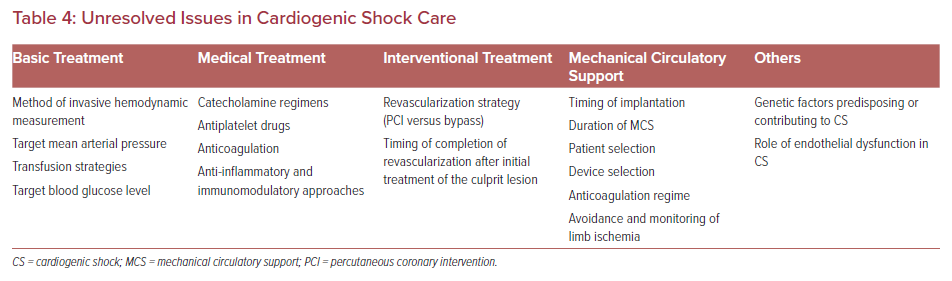
CS remains a leading cause of death in patients with acute cardiac diseases. Despite a considerable number of studies in the field of CS, major areas of care are poorly understood. Table 4 provides an overview of unresolved issues in CS care.
The key question in management of CS is how to interrupt the vicious cycle of CS progression. With AMICS, revascularization is crucial; nonetheless, evolving CS has to be treated symptomatically. Inotropic and vasopressor support has shown to have limited benefit or even cause harm in CS.77,78 The effect MCS can have on stabilizing hemodynamics has led to its widespread use, but is yet to be shown to improve clinical outcomes that matter to patients and caregivers.
Although we have seen an increasing number of studies in the field of MCS in recent years, the optimal strategy remains unclear. Results to date suggest that stratification is necessary and there is no one-size-fits-all solution.
Large RCTs have to answer questions on rational selection of patients, the best modality of MCS in different clinical circumstances, the efficacy of combining different types of MCS, and the optimal timing for implementation of MCS.
Furthermore, basic science needs to help improve our understanding of the underlying mechanisms and importance of areas such as immunomodulation, endothelial function, and genetics.
Difficulties in designing and performing high-quality clinical trials in this very sick patient population complicate the evolution of systematic and consistent evidence-based CS management protocols. Despite this, well-designed trials in clearly-defined CS patient populations must now be established.79