Transcatheter aortic valve replacement (TAVR) is an established method for the treatment of severe aortic stenosis in inoperable, high-risk and intermediate-risk individuals. Recently, the indication for TAVR was expanded to include low-risk individuals based on the PARTNER 3 and Evolut Low Risk Trials.1,2 These trials demonstrated equivalent outcomes in terms of mortality and stroke between transcatheter and surgical valve replacements.
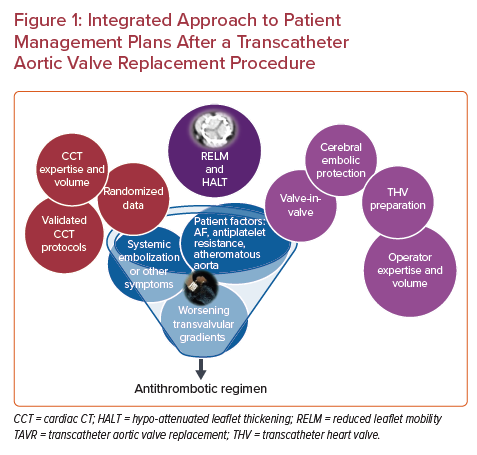
To date, there remains no consensus on the optimal antiplatelet or antithrombotic regimen to reduce stroke and ensure valve durability (Figure 1). The American College of Cardiology (ACC) valve guidelines currently recommend aspirin or clopidogrel monotherapy in the periprocedural period in patients without an indication for anticoagulation therapy. Then, for the first 3–6 months after TAVR, either single or dual antiplatelet therapy (DAPT) is recommended, based on the individual bleeding risk. After the initial 3–6 months, single antiplatelet therapy is recommended long-term. For those with an indication for anticoagulation, the guidelines recommend aspirin or clopidogrel monotherapy only (no anticoagulation) in the periprocedural period. In the first 3–6 months post-procedure, anticoagulation with or without single antiplatelet therapy is recommended, depending on the risk of bleeding. After the initial 3–6-month post-procedure period, oral anticoagulation monotherapy is recommended.3
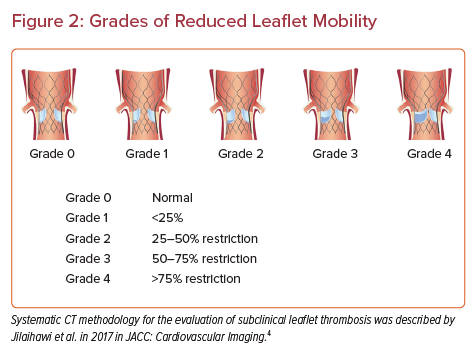
Cardiac CT (CCT) evaluation of the prosthetic valves is often employed to detect thickening, hypo-attenuated leaflet thickening (HALT) and reduced leaflet mobility (RELM). These findings have been described in the literature and assist in grading the degree of bioprosthetic leaflet thickening and dysfunction following TAVR. Figure 2 provides an illustration of the grading described by Jilaihawi et al.4 The PORTICO IDE study, RESOLVE registry, the SAVORY registry and the PARTNER 3 CT sub-study were all trials that assessed leaflet motion and HALT using CCT in those who were asymptomatic, as well as those who suffered a stroke after TAVR. In these trials, there was poor correlation of the CCT findings with clinical outcomes in particular stroke and valve function.5–8 The GALILEO 4D sub-study included patients without an indication for long-term anticoagulation who had undergone successful TAVR.9 A rivaroxaban-based antithrombotic strategy was adopted and found to be more effective than an antiplatelet-based strategy in preventing subclinical leaflet-motion abnormalities. However, in the main GALILEO trial, the rivaroxaban-based strategy was associated with a higher risk of death and thromboembolic complications as well as a higher risk of bleeding than the antiplatelet-based strategy.10 Accordingly, it was terminated early and direct oral anticoagulants are currently not routinely recommended after TAVR.
The objective of this single center observational study is to examine the clinical, echocardiographic and CCT findings of all patients receiving DAPT after their TAVR procedure. The aim is to identify any clinical correlation of this regimen with the incidence of HALT or RELM and define the role of CCT in the evaluation of patients with worsening gradients or symptoms.
Methods
This is a single center retrospective descriptive study of all patients who underwent TAVR using the Sapien XT, Sapien 3, CoreValve, Evolut R or Acurate Neo valve between 2017 and 2019. Patients above the age of 18 undergoing transfemoral TAVR were enrolled. For those who successfully completed the TAVR procedure and were discharged from the hospital, a follow-up CCT was arranged at 6 months and they were included in the final analysis. As part of the center’s protocol, all patients received a DAPT regimen consisting of a loading dose of aspirin 300 mg and clopidogrel 600 mg the day before the planned procedure and aspirin 81 mg daily and clopidogrel 75 mg daily for 1 year followed by a single antiplatelet therapy consisting of aspirin 81 mg daily. Those with AF as an indication for anticoagulation were placed on a direct oral anticoagulant (apixaban 5 mg twice daily or rivaroxaban 20 mg daily) alone on the first day post-procedure (if no bleeding complication occurred) and continued indefinitely. Patients on anticoagulation for reasons other than AF, specifically the presence of a mitral mechanical valve prosthesis, were excluded. Other exclusion criteria were valve-in-valve TAVR, aspirin allergy, bleeding or thrombotic disorders, or those requiring alternate access.
Baseline demographics, characteristics, Society of Thoracic Surgeons (STS) score for mortality and echocardiographic findings and clinical outcomes were compiled through a chart review. Patients were followed up clinically and echocardiographically at 1 month, 3–6 months and 1 year as part of the routine protocol. The echocardiographic findings at baseline and 6-month follow-up consisted of left ventricular function, hypertrophy, valve gradient, valve regurgitation and other valve disease. Patients were also assessed clinically for symptoms as part of their routine evaluation on the day of the follow-up echocardiogram. Those who completed 6 months of follow up underwent CCT examination of the aortic valve prosthesis.
Cardiac CT Protocol
The CCT findings at follow up primarily focused on the bioprosthetic valve leaflet thickness, attenuation and mobility, and paravalvular leak. Examinations were acquired in the caudocranial direction using a dual-source CT system (Siemens Somatom Definition Flash, Siemens Healthcare). Retrospective ECG-gating with high-pitch helical (3.4) acquisition was employed. Contrast media (Ultravist 350 mg/ml) was administered as a 10 ml test bolus followed by 50 ml for all scans. Saline flush (50 ml) was the employed chaser. Heart rate reduction with β-blockade (metoprolol) was prescribed for the majority of patients to achieve a rate <65 BPM. Standard collimation of 128 by 0.750 mm, a gantry rotation time of 280 ms, and a tube current ranging from 450 to 750 mA with a fixed tube potential of 100 kV (BMI <30 kg/m2) or 120 kV (BMI >30 kg/m2) was selected for the acquisition protocol. Current modulation to 20% of maximum was used when possible for dose reduction. Images were evaluated on the Syngo Via workstation using multiplanar reformatting (slice thickness 0.75 mm), maximal intensity projections (slab thickness of 3–5 mm) in short axis, two chamber long axis and four chamber long axis as well as 3D volume rendered reconstruction of the aortic valve using automated aortic valve and prosthetic valve views, as well as 4D volume rendering. The maximal leaflet excursion was assessed using at least 10–15 phases. The distance between the frame margin and the maximally open leaflet tip of the thickened leaflet and the center of the frame was measured to determine the excursion.
HALT was classified in accordance with previously published definitions as follows: present requiring further assessment of impaired leaflet mobility; absent; or indeterminate (uninterpretable scan).4 Similarly, RELM was classified as follows (Figure 2): Grade 0, normal; Grade 1, <25%; Grade 2, 25–50% restriction; Grade 3, 50–75% restriction; Grade 4, >75% restriction.4
Statistical Analysis
Continuous variables are presented as mean ± SD. Categorical variables are presented a percentage.
Patient and Public Involvement
The involvement of patients was only through their chart review with no direct impact on their institutionally determined management plan.
Results
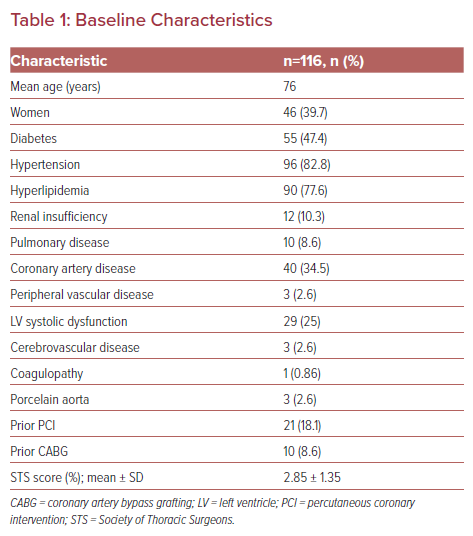
A total of 116 patients were included in this study. The mean age was 76 years and 39.7% were women. The baseline characteristics are summarized in Table 1. Of note, three patients had a prior history of a cerebrovascular accident and three had peripheral vascular disease that required revascularization in the past. At baseline, five patients had AF and were receiving oral anticoagulation (all were direct antithrombin inhibitors). The mean STS score was 2.85 ± 1.35. The baseline mean ejection fraction was 54.3% (range 30–75% ), mean gradient 50 mmHg and mean peak gradient 80 mmHg. DAPT was assigned to 111 of the total group, with five requiring anticoagulation for AF (four rivaroxaban and one apixaban).
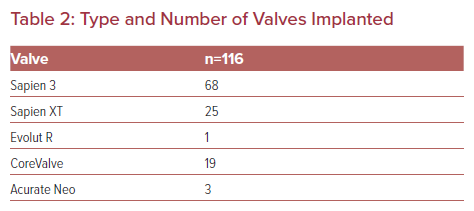
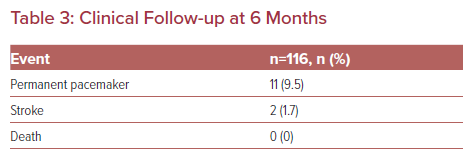
The valves implanted are shown in Table 2. The aortic valve annulus ranged from 18 mm to 34 mm (mean 24.55 mm). There were two cerebrovascular events recorded in this low-risk cohort. The two strokes occurred during the index hospitalization; both were ischemic events and both patients were receiving DAPT. One was a watershed infarction due to profound hypotension during the procedure. The second occurred within the first 24 hours of the procedure and was deemed embolic. There were no mortalities recorded in this cohort during the initial follow up before a CCT was performed or during the subsequent 6 months (Table 3).
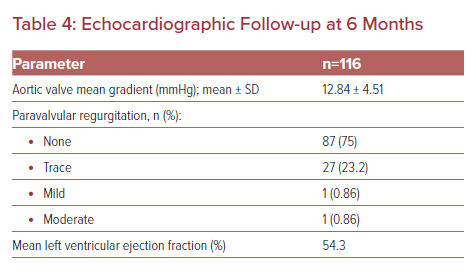
Echocardiographic findings at 6-month follow-up are summarized in Table 4. Mean gradient was 12 mmHg. One patient had an increased mean gradient of 40 mmHg at 6 months. This patient was receiving DAPT that was subsequently changed to rivaroxaban. Incidentally, his CCT showed mild HALT and 25–50% restricted excursion. He was asymptomatic and had no clinical evidence of stroke.
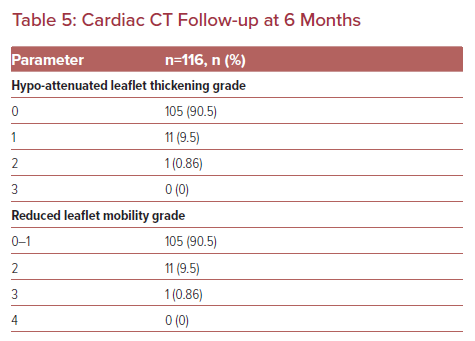
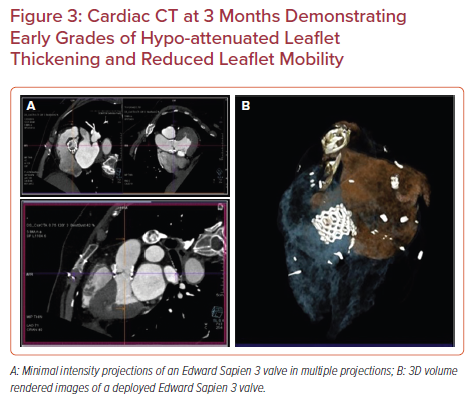
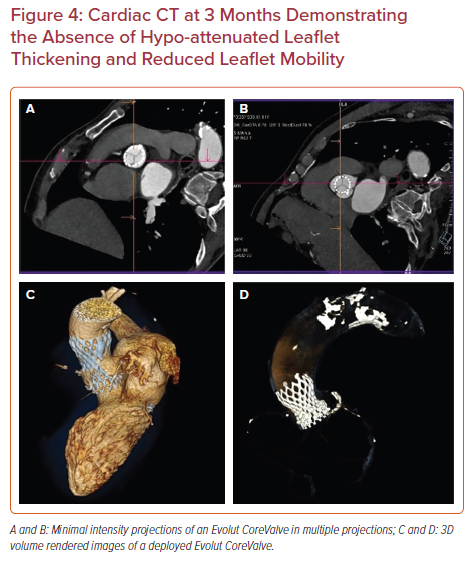
The CCT images obtained for all 116 patients were interpretable with very little artifact registered. HALT was detected in 11 of the total patients enrolled (Table 5, Figures 3 and 4, and Supplementary Figures 1 and 2). Ten of the 11 patients did not have accompanying RELM or increase in valve gradient on echocardiography. None of the 11 patients had any symptoms or clinical parameters of concern. The one patient with thickening and limited mobility with an elevated mean gradient across the aortic valve was converted to rivaroxaban therapy. A follow-up echocardiogram and CCT 3 months later demonstrated improved gradient (14 mmHg) and a significant reduction in HALT.
Discussion
The cohort reported in this study is a low-risk cohort with a mean STS score of 2.85. Nevertheless, 47.4% had diabetes and 82.8% had hypertension, which places them at risk for stroke. The population in this study is representative of the general population treated in the region at a high volume TAVR center with a 10-year-old TAVR program. The protocols adopted are also representative of most practices in the region with DAPT being the standard of care. DAPT was assigned to 111 of the total, with only five requiring anticoagulation (four rivaroxaban and one apixaban) for underlying AF. None of the five receiving anticoagulation had leaflet abnormalities on CCT. This number is too small to draw any meaningful conclusions on the role of oral anticoagulation for the treatment of newly implanted TAVR valves, particularly direct oral anticoagulants. The only available data for direct thrombin inhibition is that provided by the GALILEO 4D trial, which was terminated early because of excessive mortality.9 Other ongoing studies include the ENVISAGE and ATLANTIS trials examining oral anticoagulation post TAVR in individuals with underlying AF.11,12 The POPular TAVI study concluded that among patients who underwent TAVR and had an indication for long-term anticoagulation, oral anticoagulation alone was associated with a reduction in all bleeding and procedural bleeding compared with oral anticoagulation plus clopidogrel. Oral anticoagulation alone compared with oral anticoagulation plus clopidogrel was noninferior with respect to major adverse ischemic events. However, only 24% were treated with a direct oral anticoagulant.13
As for antiplatelet regimens, the ARTE trial randomized 222 patients to aspirin (80–100 mg/day) plus clopidogrel (75 mg/day) or aspirin alone. The rate of major or life- threatening bleeding events at 3 months was higher in the DAPT arm and there was no difference between the two arms in the rate of ischemic stroke, MI or death.14 The SAT-TAVI study evaluated 120 patients undergoing TAVR. Subjects were randomly assigned to aspirin or DAPT (aspirin plus clopidogrel 75 mg or plus ticlopidine 500 mg). There was no difference between the two arms in the rate of ischemic stroke (DAPT 1.7%; SAPT 1.7%; p=not significant).15 Based on such studies, DAPT is currently the regimen employed by the majority of TAVR centers worldwide and is the currently adopted recommendation of the ACC valve guidelines.3 Our cohort did not display any increase in bleeding or ischemic events with a DAPT regimen.
The role of CCT in the evaluation of TAVR prosthesis is still not well understood. CCT is a powerful tool to detect leaflet dysfunction and thickening with a high positive predictive value and sensitivity. Nevertheless, there is poor correlation with clinical events. In our cohort, there were only two cerebrovascular events, of which only one was ischemic and occurred eight months after the TAVR procedure. This patient did not have any HALT or RELM detected on CCT. To date, all studies evaluating leaflet thickening and anticoagulation found poor correlation between anticoagulation regimens, HALT symptoms and valve function (the PORTICO IDE study, RESOLVE registry, SAVORY registry and the PARTNER 3 C Trial).5–8 In our study, all patients underwent a CCT to evaluate the leaflets. The CCT was interpretable and provided accurate information. In the majority of patients, >90%, DAPT was sufficient with no evidence of leaflet dysfunction by CCT. In those with leaflet thickening and limited leaflet mobility, the majority had no symptoms and only one had a high mean gradient. This patient had grade 3 HALT and a high mean gradient, but did not develop any symptoms or any clinical ischemic events. The patient was receiving DAPT that was switched to rivaroxaban. Although the gradient decreased by echocardiography and the grade of HALT decreased, he did not express any change in symptoms or overall activity. In the GALILEO 4D trial patients underwent a CCT at 3 months in both the rivaroxaban (n=115) and DAPT (n=116) arms. Similar to our study, there were too few events to permit any conclusion on the impact of HALT and RELM on clinical outcomes. However, what was notable in that study was that there were fewer thickened leaflets with restricted mobility in the rivaroxaban arm compared with the DAPT arm.9
The incidence of HALT by valve type cannot be analyzed based on this small cohort. Finally, cerebral embolic protection devices were not used in any of the patients enrolled in this study. Hence, the incidence of stroke was not impacted by use of such devices. Of note, any potential benefit of embolic protection would be in the immediate post-procedure period and not the intermediate term follow-up captured in this study, which was 6 months.
CCT is an accurate tool to detect and grade leaflet thickening and restricted mobility in TAVR prosthesis.6 At this time, the detailed information provided by CCT is required only if patients are symptomatic (especially ischemic stroke) or the gradients on echocardiography have increased. Implications on the management once HALT and RELM are detected on CCT remain uncertain. Nevertheless, the majority of clinicians would opt to switch antiplatelet regimens to anticoagulation if a significant increase in gradient is associated with HALT/RELM. Based on the current body of CCT evidence generated by studies including this one, DAPT appears to be a reasonable initial regimen post TAVR in those without an indication for anticoagulation. For those undergoing valve-in-valve procedures, suffer systemic embolizations or have sustained elevation of gradients, CCT cannot define the antithrombotic regimen based on the currently available evidence nor is it supported by the guidelines.
Limitations
This is a relatively small single-center study that may not be representative of other center practices. Additionally, this is a low-risk population and the role of both DAPT and CCT at 6 months may not be applicable for a higher-risk population requiring closer and longer follow-up and more intensive therapy. Finally, CCT protocols and expertise may not be widely available to permit wide scale use of CCT for routine follow up. However, these are real-world data representative of the type of patients undergoing TAVR, the standard regimen of DAPT and the utility of CCT in most tertiary care centers in this region.
Conclusion
Our study demonstrates the ability of CCT to detect and grade leaflet thickening and restriction post-TAVR in low-risk individuals treated with DAPT. However, the role of CCT in guiding antithrombotic regimens cannot be ascertained from this study. Additional larger scale randomized studies determining the ability of CCT to impact prognosis and to compare the efficacy of different regimens in both symptomatic and asymptomatic patients are necessary, particularly in high-risk populations.