Aortic dissection (AD) is a life-threatening condition characterized by a split between the tunica intima and tunica media of the aorta.1 The clinical classification of AD is based on the onset of symptoms and the anatomical location of the split. The condition is relatively uncommon; however, it has a high mortality rate.1 Because there is no specific examination to diagnose AD, it is rarely diagnosed at an early stage. It can affect a variety of groups of people, including men, women, those who are pregnant, and any age group. Early diagnosis is made by observing the signs and symptoms, which can also be common to many other cardiovascular diseases, along with imaging. Understanding the risk factors for AD is important for disease management. The aim of this review is to describe the patient characteristics, drugs, and medical procedures that may contribute to an increased risk of AD, raising awareness of this life-threatening condition.
Classification of Aortic Dissection
Up to 40% of patients with acute AD occurring in the ascending aorta die immediately.1
AD is classified based on the time of appearance of symptoms and the anatomical presentation. The onset of the disease can be characterized by hypertension or neurological symptoms, such as transient or persistent thoracic or abdominal pain. Regarding the time of appearance of symptoms, the International Registry of Acute Aortic Dissection (IRAD) classification by Booher et al. describes four time periods: hyperacute (onset within 24 hours), acute (onset within 2–7 days), subacute (onset within 8–30 days), and chronic (onset in >30 days).2
In 1965, Debakey et al. described the anatomical classification of AD.3 In this classification, AD is differentiated according to the point of the initial tear and its extension. In Type I, the initial tear is located in the ascending aorta and can extend at least to the aortic arch. In Type II, the initial tear is limited to the ascending aorta. In Type III, the initial tear occurs in the descending aorta. Type III is divided into two subtypes: Type IIIA, where the dissection is confined to the thoracic aorta; and Type IIIB, where the dissection can extend into the abdominal aorta.
In the Stanford classification of AD, the Debakey classification is simplified depending on whether or not the ascending aorta is involved in the dissection.3 In Stanford A (proximal), the dissection affects the ascending aorta and includes both Debakey Type I and II. In Stanford B (distal), the dissection does not include ascending aorta. Dissection limited to the aortic arch, described in 2018 by Czerny et al., is referred to as non-A non-B AD.4
Risk Factors for Aortic Dissection
AD has been described as occurring in the area where the pressure that is applied on the aorta is greater than its strength.5
Age and Sex
The incidence of AD is higher in older patients than in younger patients. In young patients, AD is typically associated with connective tissue disorders (CTDs), such as Marfan syndrome (MFS), Ehlers-Danlos syndrome (EDS), and Loeys-Dietz syndrome (LDS). In older patients, it is most often associated with underlying cardiovascular diseases (CVDs), such as atherosclerosis and hypertension, and aneurysms.6 The distribution of the disease between men and women is somewhat disproportionate: men present a higher risk of type A AD than women. Women with AD are much older.7 Meanwhile, some studies show that men may be more likely to develop Type A acute AD than women at young ages.8
Aneurysm
Aneurysm is characterized by artery dilation of at least 1.5 times the normal size. It is associated with aging, smoking, and atherosclerotic disease. Aortic aneurysm (AA) is three to four times more prevalent in men than women.9 It can be grouped according to its location as abdominal AA and thoracic AA. Thoracic AA is a life-threatening condition because of its tendency to rupture and progress into AD. Both have genetic factors that are important in their respective pathogenesis pathways. Thoracic AA is the most dangerous type of AA, not only because of the location (the aortic root), but also because it can evolve into dissection. It is strongly inherited; to date, 11 genes have been found to contribute to thoracic AA pathogenesis.10 FBN1 and MFAP5 are two genes responsible for the stability of the aortic wall via the formation of microfilaments. Any disruption and modification of these genes can weaken the aortic wall. Mutations in ACTA2, MYH11, MYLK, PRKG1, or MAT2A disrupt smooth muscle cell contraction and metabolism, which can lead to weakness of the wall. The transforming growth factor (TGF)-β and SMAD pathways, involving TGFBR1, TGFBR2, TGFB2, and SMAD3, are responsible for maintaining vascular function. Any mutations that may result in disruption of these activities may promote aneurysm or dissection.11
Congenital Disorders
CTDs are a group of congenital disorders responsible for the malformation of blood vessels, leading to their weakness. When present, CTDs are responsible for early AD and aneurysm, explaining why these risk factors are mostly seen in young patients with AD. Inherited CTDs that can increase the patient risk of developing AD include MFS, EDS, and LDS. The common factor among these inherited disorders is that they all contribute in some way to the abnormal formation of the structure of blood vessels, especially the aorta. The identification and the monitoring of patients with CTDs will not only reduce the complications of the dissection but also decrease mortality rates.6
Marfan Syndrome
Marfan syndrome is an autosomal dominant condition, recognized by its skeletal, cardiovascular, ocular, lung, skin, and nervous system manifestations. Its diagnosis is based on the Ghent criteria. The Ghent nosology was revised in 2010, placing more emphasis on the cardiovascular manifestations of MFS. The cardiovascular Ghent criteria for MFS diagnosis can be summarized as follows:12
- In the absence of family history, the diagnosis can be made in the presence of aortic root dilation and at least one of the following: ectopia lentis, FBN1 gene mutation, and/or a positive systemic score.
- In the presence of family history, the diagnosis can be made if any of the following are also present: aortic root dilation, ectopia lentis, and/or a positive systemic score.
The risk of developing acute AD is amplified with MFS. The IRAD analysis of 6,424 acute AD patients from January 1996 to May 2017, including 258 people with MFS, confirmed that patients with AD associated with MFS were young with fewer comorbidities and a larger diameter of the aortic annulus and root.13
FBN1 encodes fibrillin-1, which forms microfibrils. Microfibrils act as tissue support and storage of TGF-β. Any FBN1 gene mutation that occurs on chromosome 15q21.1 reduces the amount of fibrillin 1. Structural alterations or fibrillin instability lead to structural changes in vascular smooth muscle cells, and vascular tissue weakness exposes the patient to AD.14
Ehlers-Danlos Syndrome
Initially described by Edward Ehlers in 1898 and Henri Alexandre Danlos in 1908, EDS is a heterogeneous group of inherited CTDs.15 Unlike the 1998 classification by Beighton et al., which classified EDS into six subtypes, the 2017 EDS international classification by Malfait et al. recognizes 13 subtypes. Among the 13 EDS subtypes, vascular EDS (vEDS) has a high risk of causing AD.16 In vEDS, mutations in the COL3A1 gene, which encodes collagen type III, are responsible for lower collagen levels. Collagen fibers are one of the most important components of connective tissue, and a mutation that induces the reduction of collagen fiber may cause vascular smooth muscle weakness, with thinning of the intima and media, which leads to aneurysm and, potentially, AD.17
Loeys-Dietz Syndrome
LDS is an autosomal dominant CTD characterized by arterial tortuosity, hypertelorism, a wide or split uvula, and aortic root aneurysm. Among all CTDs, LDS is one of the most recently described. The vascular manifestations of LDS (AD and arterial aneurysms) are more severe than those in MFS. Unlike MFS, in LDS the aneurysm often occurs at the aortic root, and the rupture can occur at an early age – a case of AD associated with LDS has been reported in a 3-month-old infant – with even smaller aortic dimensions.18 Like MFS, LDS is a rare CTD caused by the mutations in genes affecting the TGF-β (TGFBR1, TGFBR2, SMAD3, TGFB2, and TGFB3) pathway. These mutations induce the alteration of signaling in the TGF-β family, which can interfere with the collagen production responsible for connective tissue formation. The direct cardiovascular consequence is the weakness of the blood vessels leading to aneurysm and AD.18–20
Congenital Bicuspid Aortic Valve
Congenital bicuspid aortic valve (BAV) is an inherited autosomal dominant disease associated with numerous CVDs including aortic valve diseases (regurgitation and stenosis), infective endocarditis, aortic dilation, aneurysm, and AD. BAV is a congenital heart defect in which the aortic valve leaflet changes from tricuspid to bicuspid. The link between BAV and such CVDs is the reason for the associated high mortality rate.21 BAV is present in approximately 1–2% of the population and has a male:female ratio of 3:1.22,23 It was identified by the Sievers classification based on the number and location of the raphe, but a new nomenclature and consensus classification has recently been proposed.24 First in this classification is fused BAV, whereby two or three of the cusps are fused; it represents 90–95% of all BAV cases. The second is two-sinus BAV. It is uncommon, representing 5–7% of cases. The third is partial-fusion BAV, which has an unknown prevalence because it has only recently been recognized.
A group of genes, including NOTCH1, AXIN1, EGFR, ENG, GATA5, NKX2-5, NOS3, PDIA2, and TGFBR2, are associated with BAV; variants associated with BAV in men (EGFR rs533525993, and TEX26 rs12857479) and women (NOTCH1 rs61751489, TGFBR2 rs1155705, and NKX2-5 rs2277923) have also been described.23 The bicuspid leaflets are known to cause hemodynamic dysfunction that leads to enlargement of the aorta, aneurysm, and dissection.25,26 However, a community-based study of 416 patients with confirmed BAV revealed that only two of the 416 patients progressed to AD.27
Coarctation of the Aorta
Coarctation of the aorta is a congenital heart defect characterized by narrowing of the aorta, typically near the ligamentum arteriosum and the origin of the left subclavian artery. It has an incidence rate of four in 10,000 live births; coarctation of the aorta – if untreated – has a mean age of death of approximately 34 years with complications such as AD, aortic rupture and many more.28 There are two types of aortic coarctation.29 Critical aortic coarctation represents about 60% of all aorta coarctation and symptoms occur within 2 months of birth. Asymptomatic coarctation occurs later in life and is associated with upper-limb hypertension.
Although coarctation of the aorta is a congenital disorder, it is difficult to diagnose at birth. Unfortunately, it sometimes remains undiagnosed because symptoms occur at least around 2 months for the critical type and later for the asymptomatic type. The narrowing of the aorta due to coarctation raises the upper-body blood pressure, leading to hypertension.30
Turner Syndrome
Turner syndrome (TS) is a condition that affects females and is characterized by a partially or completely missing X chromosome. Its prevalence is 1 in 2,500 newborn females. CVDs such as BAV, coarctation of the aorta or abnormalities of the arteries in the head and neck are major issues in individuals with TS. The risk of AD is more severe in TS than in the general population and the AD occurs at a young age.31–33
Hypertension, Atherosclerosis, and Smoking
Hypertension contributes to the deterioration of arterial walls. Approximately 65–75% of AD patients also have hypertension. This is well explained by Dong et al., who performed a study in 838 confirmed acute AD patients, finding that 585 acute AD cases were associated with hypertension.34 AA and AD both represent target organ damage of systemic arterial hypertension, so controlling blood pressure is highly important in preventing progression to AD.35
Hypertensive crisis is an unusual acute and severe increase in blood pressure, with systolic blood pressure >179 mmHg and diastolic blood pressure >109 mmHg. It can be divided into hypertensive urgency and hypertensive emergency, with the difference between the two being the presence of target organ damage in the latter; the target organ can be the brain, kidney, or heart. AD may classically present as hypertensive emergency.36
In the aorta, atherosclerosis can progress into an ulcer that penetrates the aortic wall – the penetrating atherosclerotic ulcer. This usually develops in patients with advanced atherosclerosis, and can progress to dissection or rupture.37
Tobacco smoking accelerates atherosclerosis and is a major risk factor for aortic aneurysms and dissections.
Inflammatory Disease
Aortic smooth muscle cells are the main components of the tunica media and are responsible for aortic wall integrity and arterial wall remodeling. Any degradation of this structure can directly alter the aortic wall and lead to CVD. Interleukin 18 is a pro-inflammatory cytokine related to CVDs, such as AD. Plasma interleukin 18 increases in patients with AD.38 Furthermore, according to IRAD, certain inflammatory diseases that affect the integrity of the blood vessels – such as aortitis, giant cell arteritis, Takayasu’s arteritis, systemic lupus erythematous, syphilitic aortitis, and rheumatoid arthritis – can cause AD if not well treated. Macrophage hypoxia inducible factor 1α increases the severity of blood vessel dilation in AD.39 Macrophages are responsible for the abnormal increase in reactive oxygen species, protease, and cell adhesion molecules underlying apoptosis and the degradation of aortic smooth muscle cells.40
Obesity
Compared with healthy people, those with obesity are more predisposed to develop acute AD, with even more respiratory complications, such as acute lung injury. In some patients with obesity, chronic inflammation (adipose tissue releases inflammatory cytokines) and oxidative stress are common. Fat accumulation and increased BMI contribute to elevated oxidative stress, with inflammation as a direct consequence.41 BMI increases with peripheral vascular adipose tissue, which can cause inflammation. BMI-related inflammation explains the association between obesity and aortic disease, such as aneurysm and dissection. Inflammatory molecules, such as proteases, inflammatory cells, and inflammatory cytokines, responsible for aortic degradation are believed to originate from the peripheral vascular adipose tissue surrounding the aortic wall. It is important to note that BMI differs according to the geographic area: the prevalence of obesity in Asian populations is lower than that in western populations. As such, obesity-related AD is more common in western than Asian regions.42
Pregnancy and Postpartum
There is a risk of AD developing during or immediately following pregnancy. This is closely related to genetic predisposition, such as MFS, LDS, vEDS, BAV, and TS, but – in rare situations – it can occur in the absence of these risk factors. MFS, LDS, vEDS, BAV, and TS increase the fetal mortality rate related to AD in pregnancy. According to Prendes et al., AD incidence during pregnancy is 14.5 per million versus 1.24 per million for non-pregnant women.43 When AD occurs during pregnancy, it is considered a catastrophic event because it can be fatal for both mother and child. Hemodynamic and hormonal changes (increased progesterone and estrogen levels) contribute substantially to aortic-wall modification, leading to an increased aortic diameter. AD in pregnancy often occurs in the third trimester or postpartum period. Preeclampsia can also be a factor in aortic-wall modification in pregnant women.43,44 However, it is rare, with 0.1–0.4% of all AD cases related to pregnancy, and affects 5.5 per million women during pregnancy and postpartum.45 AD of Stanford A type is most common in pregnant women in the third trimester, while Stanford B type is mostly present postpartum.46
Drugs and Illicit Substances
Sildenafil and Tadalafil
Sildenafil inhibits phosphodiesterase type 5 (PDE5), an enzyme in the blood vessel walls that regulates vascular smooth muscle cells and blood flow. PDE5 inhibitors blocks the effect of phosphodiesterase, treating erectile dysfunction by relaxing blood vessels and increasing blood flow. Historically, sildenafil was designed to treat hypertension and angina pectoris, but did not achieve its treatment goals. However, it was found that it could induce penile erection. In 1998, the Food and Drug Administration approved the use of sildenafil to treat erectile dysfunction.47,48 Sildenafil became the first oral pill for erectile dysfunction in the US. It is generally associated with minor side-effects, such as headaches and flushed skin. Nevertheless, some cases of AD have been associated with sildenafil, because it is a vasodilator and can decrease aortic stiffness.49 These properties can induce aortic intimal tearing. Therefore, this medication can be a risk factor or an aggravating factor for AA and AD.50
Tadalafil is another PDE5 inhibitor used to treat erectile dysfunction. It has the same adverse effects on the aortic wall and can also lead to AD. The dissection occurs at the time between the peak plasma concentration (30–120 minutes) and half-life (3–5 hours) of drug administration.51
Cocaine
Cocaine can be used medically to relieve pain, but it is most often used as an illicit drug. The use of cocaine leads to a variety of complications, including acute hypertension, arrhythmia, coronary spasm, and MI.52 Cardiovascular risks are closely linked to cocaine blood levels.
There have been numerous cases of AD related to cocaine use or abuse. According to IRAD, cocaine use was reported in 1.8% of all cases of AD, with a higher incidence of acute Stanford A type AD, and patients with cocaine-related AD are usually young men. The time between cocaine intake and the dissection is approximately 1 hour.53 Cocaine is listed among both predisposing and aggravating factors for AD.54 The effects of cocaine in the cardiovascular system are numerous.55 They include hypertension and myocardial ischemia/infarction due to the increase in myocardial oxygen demand and coronary vasoconstriction. Cocaine also induces vascular smooth muscle cell apoptosis that leads to blood vessel weakness and endothelial dysfunction that decreases aortic strain and stiffness.
Medical Procedures: Coronary Artery Bypass Graft Surgery and Cardiac Catheterization
Iatrogenic AD (IAD) is the presence of AD as a complication of a cardiac procedure. It is a rare and potentially fatal condition that can occur during or after the procedure. During coronary artery bypass grafting (CABG) , the cannulation site and the side clamp are the most common causes of IAD.56,57 Like CABG, cardiac catheterization can also lead to IAD. IAD might be associated with a high risk of perioperative morbidity in elderly patients who undergo CABG or cardiac catheterization and have pre-existing aortic pathology.58
Conclusion
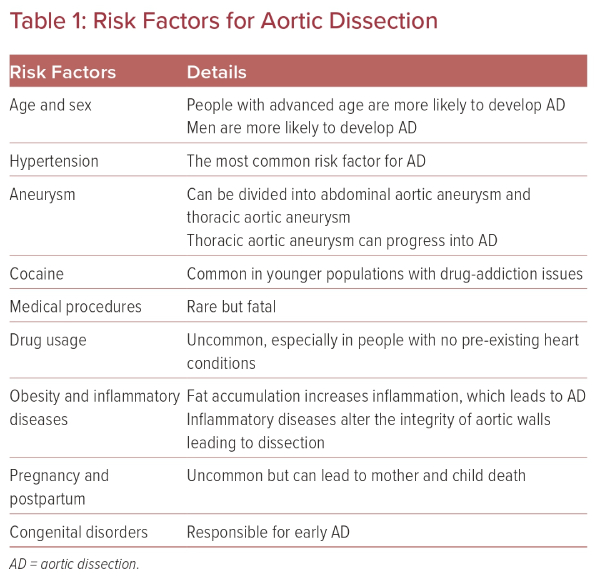
AD is an uncommon life-threatening condition characterized by a tear in the tunica intima of the aorta. Despite progress in medicine and cardiovascular surgery, AD remains one of the most serious emergency conditions. The dissection can affect any segment of the aorta and can occur at any age. The risk factors described in this review play a key role in AD occurrence and development (Table 1). Among them, hypertension is the most common and is mostly found in elderly patients. This is in contrast to CTDs, which are usually found in young patients presenting with AD. The occurrence of AD during pregnancy with or without pre-existing CTD is a catastrophic event. Having the knowledge that pregnancy might be a risk factor for AD allows the specialist to set up counseling for pre-pregnancy and postpartum. AD is also strongly linked to lifestyle, and the use of illicit drugs has become a potential risk factor. IAD can occur during or immediately after a medical procedure and, while dissection caused by such procedures is rare, it is associated with a high mortality rate.
Understanding the risk factors for AD may contribute to the slowing down of progression, limiting complications of the disease, and improving patient prognosis.