The history of documented heart failure in medical literature dates back as early as the late 1700s, when William Withering recognized the therapeutic use of foxglove in patients with “dropsy”. The extract from the foxglove plant contained the cardiac glycoside digitalis, and edema – known as “dropsy” – was described in patients we now presume had the clinical syndrome of heart failure.1 Over the centuries, growing medical knowledge along with the expanding body of literature has led to an increasing understanding of heart failure, and notably awareness of the distinct clinical syndromes of heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection failure (HFpEF).
Data from the National Health and Nutrition Examination Survey estimated an incidence of 5.7 million people >20 years of age had heart failure between 2009 and 2012 in the US alone.2 Currently, the prevalence of HFrEF and HFpEF is fairly even, at about 50 %.3 Data from the Framingham Heart Study noted the 5- and 10-year mortality rates for HFpEF and HFrEF to be similar.4 In comparison to HFrEF, an increased proportion of HFpEF patients die from extra-cardiac etiologies due to the role that multiple chronic comorbidities play in the development of HFpEF.5 A study comparing clinical workload and costs in HFrEF and HFpEF found that the costs are greatest within 3 months to 1 year after heart failure hospitalizations in HFpEF patients due to the frequency of non-cardiac-related hospitalizations.6
Utilizing US Census Bureau data, the American Heart Association predicts that over 8 million people will have heart failure by 2030.7 It also predicts that the total cost of heart failure will more than double, from $31 billion in 2012 to an estimated $70 billion by 2030.7
A brief survey of the heart failure literature to date reveals essential differences in epidemiology, pathophysiology, diagnosis, therapeutic modalities, and outcomes for HFpEF when compared to HFrEF. In the current review, we focus on the unique characteristics of HFpEF.
Diagnosis
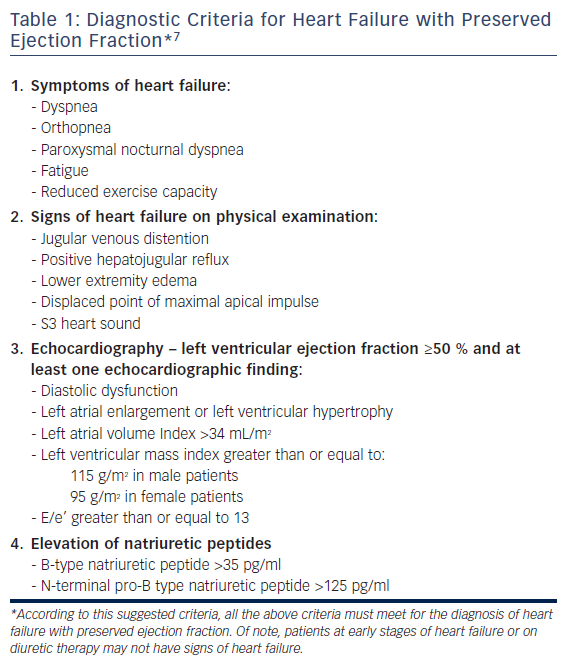
The diagnosis of HFpEF may be challenging. The diagnostic criteria for HFpEF include clinical signs and symptoms of heart failure and preserved left ventricular ejection fraction (LVEF) with echocardiographic evidence of left ventricular (LV) diastolic dysfunction or relevant structural heart disease (left atrial enlargement, LV hypertrophy) (see Table 1).8 The typical signs and symptoms of heart failure entail the presence of dyspnea, orthopnea, paroxysmal nocturnal dyspnea, fatigue, lower-extremity edema, jugular venous distention, positive hepatojugular reflux, displacement of the point of maximal apical impulse, S3 heart sound, and reduced exercise capacity.8
The LVEF cutoff for diagnosing HFpEF varies between studies, ranging from >40 % to >50 %. According to the current European Society of Cardiology guidelines, the LVEF cutoff required for diagnosis of HFpEF is ≥50 %,8,9 whereas HFrEF is defined as a LVEF of ≤40 %, leading to a phenotypic middle zone of patients with a LVEF of between 41 % and 49 % defined in the current guidelines as heart failure with mid-range ejection fracture.8,10
Assessment of brain natriuretic peptide (BNP) is part of the diagnostic criteria for HFpEF, although patients may have normal levels of this peptide.5,8,11 Anjan et al. studied BNP levels in HFpEF patients diagnosed clinically and echocardiographically. Using registry data from the Northwestern HFpEF Program, they found that one-third of patients exhibited normal BNP levels, and that obesity was an independent risk factor in this subset of HFpEF patients.5 It is postulated that there is a deficit in BNP generation, release or clearance that might be related to receptor signaling within the visceral adipose tissue, which would account for the normal or lower levels of BNP in obese/overweight HFpEF patients.5
Biochemical Pathophysiology
It is increasingly being recognized that inflammation at the systemic level, at the coronary endothelial level and within the myocardial extracellular milieu has a role in the development of HFpEF. Comorbidities such as diabetes mellitus, chronic kidney disease, anemia, and obesity contribute to systemic inflammation through an increase in the production and release of inflammatory cytokines such as interleukin 6 and tumor necrosis factor.12 The European Metabolic Road to Diastolic Heart Failure (MEDIA) HFpEF registry noted the prevalence of metabolic syndrome in their HFpEF population to be as high as 85 %.13 These comorbidities create an oxidative cellular environment, with reactive oxygen species diverting nitric oxide away from its crucial role in cardiomyocyte functioning.
This inflammatory activation leads to alterations in paracrine signaling between the coronary endothelial cells and the cardiac myocytes. This then leads to decreased levels of nitric oxide, cyclic guanosine monophosphate (cGMP), and protein kinase G (PKG), which are central components of a cascade pathway essential for cardiomyocyte functioning. PKG is a crucial protein due to its role in the phosphorylation of titin within the cardiac myocyte cytoskeleton, which decreases cardiac myocyte stiffening.3,11,14 Down-regulation of PKG leads to cardiac myocyte hypertrophy, which at the ventricular level contributes to LV chamber stiffening, causing impaired lusitropy and diastolic dysfunction which are the hallmarks of HFpEF.
Inflammation within the coronary endothelial cells also leads to increased expression and release of adhesion molecules, such as vascular cell adhesion molecule and E-selectin. These adhesion molecules induce monocytes to release transforming growth factor-beta, which leads to collagen deposition within the interstitium, further contributing to the stiffness leading to concentric LV hypertrophy (LVH).3,12,14 This highlights a fundamental difference in mechanism to the scattered foci of fibrosis leading to eccentric LVH seen with HFrEF, which is more so due to cardiac myocyte loss from ischemia with deposition of collagen to compensate for that loss.
Clinical Phenotypes
The fact that clinical trials for pharmacologic therapies have failed to improve outcomes in HFpEF patients has led to a new paradigm shift to viewing HFpEF as a heterogeneous syndrome with different phenotypic characteristics with dissimilar outcomes.15 The emergence of this concept may improve our understanding of this syndrome and may result in specific therapies for each phenotype. The main phenotypes are summarized below.
Pulmonary HTN
A common clinical phenotype is HFpEF with pulmonary hypertension (pHTN). Database comparisons have found a higher prevalence of pHTN among HFpEF patients than HFrEF, with pHTN affecting 70–80 % of the HFpEF population.3,5,16 Molecular changes leading to LVH and stiffness lead to impaired filling of the left ventricle, increasing reliance on left atrial contraction for both LV filling and left atrium (LA) emptying. As this process progresses over time, the elevated LV filling pressures are transmitted back to the LA, and compensatory augmentation in LA activity is no longer sufficient. This leads to backflow into the pulmonary circulation, which over time affects the right ventricular (RV) afterload. This pathologic cascade infers HFpEF leading to pHTN, as per the World Health Organization Group 2 pHTN classification system. However, it is now being better recognized that the reciprocity between pHTN and RV dysfunction lead to further clinical deterioration of HFpEF, causing unfavorable morbidity and mortality outcomes. Elevated LA pressures causing backflow into the pulmonary circulation lead to damage at the alveolar–capillary interface, leading to increased permeability and impairments in gas exchange. Acutely this leads to decompensation in heart failure, but chronically the elevated pressures within the pulmonary capillary networks lead to remodeling. This remodeling extends to the pulmonary arterioles and arteries, and can be irreversible. This accounts for the natural progression of pHTN and, in turn, worsening of HFpEF. Understanding the pathologic molecular and cellular changes that occur with pulmonary microvasculature remodeling can aid the application of treatment to slow the progression of these deleterious changes.
Different ways of stratifying the severity of the HFpEF–pHTN phenotype have been proposed, including the concept of “coupling” of the right ventricle and pulmonary circulation.17 Guazzi et al. describe the use of the tricuspid annular plane systolic excursion to pulmonary artery systolic pressure ratio as an indicator of RV contractile function. His group studied pulmonary artery systolic pressure measurements for the ratio both via invasive hemodynamic and Doppler echocardiographic monitoring, with noted correlation between both kinds of measurements (r = 0.69, p<0.0001).17 This study highlights the importance of not assessing pHTN–HFpEF in a vacuum and calls for concurrent evaluation of the right ventricle, which is beneficial for categorization of disease severity, prognosis and future therapy-related targets.16,17 Recognition of pHTN as one of the clinical phenotypes of HFpEF is of paramount significance when studying therapies targeting this particular pathophysiologic cascade, with controlled randomized trials determining the efficacy, safety and outcomes of these therapies.
Obesity
It is estimated that >80 % of HFpEF patients have a BMI >25 kg/m2.3 In a study comparing obese to non-obese HFpEF patients, type 2 diabetes mellitus and obstructive sleep apnea were found to be more prevalent in obese HFpEF patients. Obese HFpEF patients were found to have increased LV cavity measurements, suggesting remodeling, and increased RV chamber size, despite adjusting for obstructive sleep apnea and higher biatrial resting pressures when compared to both non-obese HFpEF and control individuals.18 The authors also noted that compared to non-obese HFpEF and control patients, HFpEF patients who were obese demonstrated elevated pulmonary capillary wedge pressures and impairments with pulmonary artery compliance during exercise. Based on Fick’s principle, oxygen consumption (VO2) = cardiac output (CO) × arteriovenous oxygen difference (Ca – Cv).13 In this study, no differences in exercise arteriovenous oxygen concentration were noted between the HFpEF and control patients. However, exercise peak VO2 was found to be inversely correlated with weight. Obese HFpEF patients also demonstrated greater degrees of chronotropic incompetence when compared to control patients.18 It is postulated that much of the inflammation at the endothelial cellular and systemic level that leads to HFpEF also causes chronotropic incompetence.3
Renal Dysfunction
HFpEF can lead to renal dysfunction via increased right heart pressures secondary to RV dysfunction and pHTN. It can also lead to decreased effective arterial volume, and therefore decreased renal perfusion though decreased cardiac output from both reduced stroke volume and chronotropic incompetence. Reciprocally, renal dysfunction can lead to the pathogenesis of HFpEF by contributing to systemic inflammation through the release of inflammatory growth factors, through the accumulation of uremic products and decreased erythropoietin levels. Increased central venous pressures leading to congestion in the renal venous system highlight the importance of diuresis in HFpEF patients with a renal dysfunction phenotype.3
Treatment
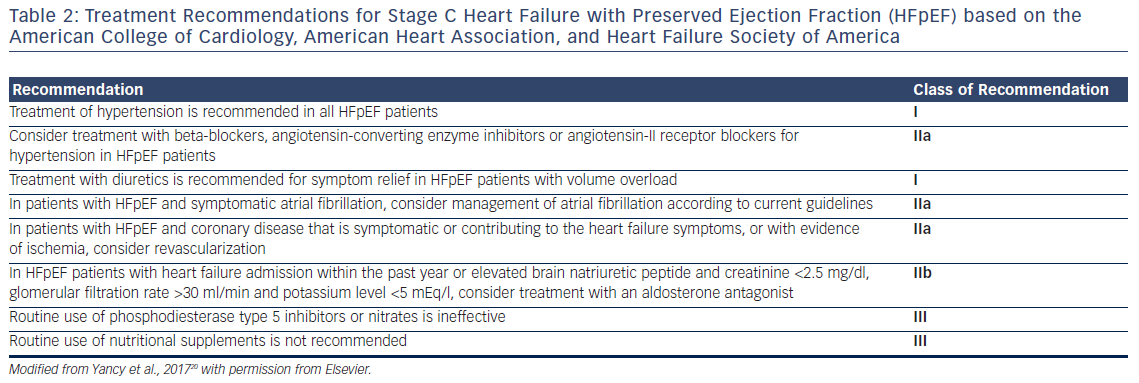
Numerous trials have aimed to evaluate various therapeutic strategies in HFpEF patients. The vast majority of these therapies have not proven to be effective, and most current pharmacologic treatments for HFpEF are used to alleviate symptoms. In this section, we review the current evidence. The recommended regimens for this patient population are given in Table 2.
Mineralocorticoid Receptor Antagonists
The renin–angiotensin system (RAS) contains many therapeutic targets for heart failure treatment. Aldosterone, a hormone implicated in the pathogenesis of myocardial and extracellular fibrosis, contributes to LV remodeling in both HFrEF and HFpEF. In the notable landmark Treatment of Preserved Cardiac Function Heart Failure with an Aldesterone Agonist (TOPCAT) trial, while spironolactone did not reduce the primary endpoints of hospitalization for heart failure, aborted cardiac arrest, or death from cardiovascular-related causes within the entire study population, there was a reduction in heart failure-related hospitalizations in HFpEF patients enrolled due to elevated BNP/NT pro-BNP levels in the Americas. The study authors considered whether regional discordances in enrollment, diagnosis, and hospitalization practices in Russia and Georgia compared to the US might have accounted for the observed outcomes rather than actual spironolactone inadequacy.19 Patients from the Americas were enrolled pretty evenly on the basis of either prior heart failure hospitalizations in the preceding year or elevated BNP levels in the preceding 60 days, while the majority of patients from Russia and Georgia were enrolled on the basis of heart failure hospitalizations. According to the current guidelines, HFpEF patients with heart failure admission within the past year or elevated BNP, and with glomerular filtration rate >30 ml/min and potassium level <5 mEq/l, should be considered for treatment with an aldosterone antagonist (class IIb recommendation).20
Phosphodiesterase Inhibitors
The nitric oxide–cyclic guanosine monophosphate (cGMP)–PKG pathway for the prevention of cardiomyocyte hypertrophy can be perturbed in many ways, including decreased bioavailability of nitric oxide or the degradation of cGMP by phosphodiesterase-5A.3,11,14 The use of phosphodiesterase-5 inhibitors, including sildenafil, prevents cGMP destruction, thus increasing the bioavailability of nitric oxide to promote vasodilatation of the pulmonary microvasculature and prevent cardiomyocyte stiffening by facilitating phosphorylation of titin through the effects of PKG.3,11,12 Phosphodiesterase-5 inhibitors selectively target the pulmonary circulation, and have fewer effects on systemic vasodilatation than endothelin receptor antagonists or prostacyclin agonists.16 Although impairments in right ventricular and pulmonary circulation coupling noted in obese HFpEF patients suggested a theoretical benefit with the use of pulmonary vasodilators in obese and pHTN HFpEF phenotypes,17 these drugs did not improve activity or quality of life and are not recommended for routine therapy according to the current practice guidelines (see Table 2).20
Angiotensin-converting Enzyme Inhibitors/Angiotensin-II Receptor Blockers
Therapeutic targets within the RAS can reduce LV remodeling and slow the up-regulation of sodium and water reabsorption at the nephron level, which can have symptomatic and long-term disease-modifying effects in heart failure patients. Additionally, angiotensin-converting enzyme (ACE) inhibitors and angiotensin-II receptor blockers (ARBs) have been shown to increase nitric oxide availability, thus preventing the pathophysiologic cascade toward myocyte hypertrophy.11 While ACE inhibitors and ARBs have demonstrated improvements in morbidity and mortality outcomes in HFrEF patients, clinical trials have failed to demonstrate significant improvements in outcomes in HFpEF patients. This highlights the need to stratify HFpEF patients into clinical phenotypic cohorts in randomized control trials to extract potentially significant outcomes.
The Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF) trial randomized elderly HFpEF patients (average age 76 years) to the ACE inhibitor perindopril or to placebo. Both a clinical diagnosis of heart failure and echocardiographic evidence of a LVEF >40 % with noted diastolic dysfunction were the diagnostic criteria for HFpEF in the trial.21 The PEP-CHF study was not able to demonstrate a statistically significant difference in all-cause mortality and hospitalizations related to heart failure between the two groups. However, there was a possible reduction in heart failure-related hospitalizations in the first year within the perindopril-treated arm of the study.21 The Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM-Preserved) trial served to assess candesartan therapy in HFpEF patients with New York Heart Association functional classes II–IV. This study found a modest decrease in the frequency of heart failure-related hospitalizations in the candesartan-treated patients compared to placebo.22
The Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE) evaluated the impact of this ARB on mortality and cardiovascular-related hospitalizations in patients >60 years of age, with an LVEF >45 %, and whom were classified as New York Heart Association functional class II–IV. They found that irbesartan therapy did not affect the study endpoints. However, it is worth noting that in the irbesartan-treated arm 39 % of patients were also taking ACE inhibitors, 28 % were taking spironolactone, and 73 % were on beta-blockers. The trial investigators proposed that patients already on multiple drugs targeting the RAS system were unlikely to demonstrate further gain with the addition of an ARB to their regimen.23
Since most HFpEF patients suffer from hypertension, ACE inhibitors or ARBs are recommended in these patients. In addition, current guidelines recommend the use of ARBs in HFpEF patients to reduce hospitalizations (class IIa recommendation) (see Table 2).20
Beta-blockers
Beta blockade has many potential mechanisms of action in the pathophysiologic processes of HFpEF. Due to up-regulation of the RAS in HFpEF, beta-blockers can curtail RAS activation by reducing the release of renin. Beta-blockers can also be utilized in HFpEF patients with atrial fibrillation or coronary artery disease.24 The Effects of Long-term Administration of Nebivolol on the Clinical Symptoms, Exercise Capacity, and Left ventricular Function of Patients with Diastolic Dysfunction (ELANDD) trial studied the use of nebivolol therapy for 6 months in patients with diastolic dysfunction and a LVEF >45 %. The trial found no statistically significant improvements in peak VO2 and walking distance during 6-min walk testing.25 The trial investigators outlined the negative chronotropic effects of nebivolol as the etiology for the reduced exercise tolerance noted in their study superimposed onto the well-known concept of chronotropic incompetence seen in HFpEF patients during exercise.18,25
Utilizing the Swedish heart failure registry, Lund et al. studied the effects of beta-blocker therapy in matched cohorts of HFpEF patients, performing a consistency analysis using control HFrEF patients on beta-blocker therapy. They found a reduction in all-cause mortality but not heart failure hospitalizations for HFpEF patients on beta-blocker therapy.26 A meta-analysis performed by Bavishi et al. utilizing 17 studies (15 observational studies and two randomized-controlled trials) found a 19 % decrease in all-cause mortality in HFpEF patients on beta-blocker therapy.27 It should be noted that meta-analyses have limitations, especially with regards to confounding variables and adjustment for covariates within the individual studies included. Current practice guidelines recommend the use of beta-blockers to control hypertension in HFpEF patients as a class IIa recommendation (see Table 2).20
Statins
In addition to the lipid-lowering properties of statins, this class of drugs has an independent mechanism that modulates redox reactions at the endothelial level. This mechanism is favorable in HFpEF patients as it helps to improve the availability of nitric oxide as part of the nitric oxide–cGMP–PKG cascade, reducing cardiomyocyte hypertrophy. This benefit has been confirmed by studies measuring levels of PKG and nitric oxide derivatives in endomyocardial biopsy tissue samples from HFpEF patients treated with statins.3,12,28
A study by Fukuta et al. that evaluated HFpEF patients on statin therapy noted a reduced mortality, even after propensity score matching their sample population.29 The study investigators attributed the improvement in survival not only to the beneficial effects of statin therapy on coronary artery disease, but also to the antioxidant and anti-inflammatory properties of statins in HFpEF. In a meta-analysis of 11 studies, Liu et al. found a 40 % decrease in all-cause mortality with statin use in HFpEF patients.30 They also noted a decrease in mortality rates at both short- (<5 years) and long-term intervals (>5 years) with statin use within the HFpEF population.30
Future Therapeutic Options: Mechanical Circulatory Support Devices
For patients with severe refractory HFpEF in whom the above therapies have provided no benefit in terms of mitigating disease progression and symptoms, durable mechanical circulatory support (MCS) devices may reduce LV filling pressures and allow decongestion and increased cardiac output. The greatest logistical challenge when implanting MCS devices in HFpEF compared to HFrEF patients is the reduced LV chamber dimensions. In a theoretical paper, Burkhoff et al. studied the role of a partial MCS device called the Synergy® system, which entailed cannulation of the LA with forward flow directed into the subclavian artery. This micropump system was theoretically found to reduce pulmonary and left atrial pressures while increasing cardiac output in HFpEF patients. Furthermore, implantation of this device consists of a minimally invasive approach, with the device placed within the subcutaneous tissue, similar to pacemaker placement.31 Further studies are needed to evaluate whether this invasive therapy will improve survival.
Another potential therapeutic option is the placement of a transcatheter interatrial shunt device in HFpEF patients with refractory symptoms on medical therapy. In the Reduce Elevated Left Atrial Pressure in Patients with Heart Failure (REDUCE LAP–HF) study, HFpEF patients demonstrated reduced pulmonary capillary wedge pressures during exercise at 6 months following implantation of the interatrial shunt device. These patients also demonstrated beneficial progress in their 6-min walk times and quality of life metrics.32
Conclusion
With the HFpEF population projected to exceed that of the HFrEF population, the study of the clinically variegated syndrome of HFpEF remains crucial. While the therapeutic modalities discussed in this review pose a theoretical and practical benefit in the management of HFpEF patients, it is essential that future randomized-controlled clinical trials utilize the many different clinical phenotypes of HFpEF in their study design. Better understanding of the role of chronic comorbidities in the pathogenesis and clinical phenotypes of HFpEF will allow for treatment at patient and population-wide levels.