The Fontan operation is a surgical repair for a broad spectrum of severe congenital heart defects in which the restoration of the function of both ventricles is not feasible. The concept of the Fontan circulation has continuously evolved over the course of five decades with substantial technical modifications. Still, the main idea of this staged procedure is to use a single ventricle as a systemic pumping chamber for both systemic and pulmonary circulation. Lacking a power source to propel blood through the pulmonary circulation is an inherent feature of Fontan physiology, with passive forward flow of deoxygenated blood directly from the caval veins into the pulmonary arteries.1 To date, the Fontan operation remains one of the most significant achievements in pediatric congenital surgery, enabling children who previously had a slim chance of survival, to survive and reach adulthood. However, the burden of long-term complications and unfavorable residual lesions pose a significant challenge for congenital cardiologists.2
After decades of follow-up, it has become clear that Fontan failure is not only circulation failure but rather a systemic condition associated with the Fontan circulation involving multiorgan damage.2,3 With chronic systemic venous congestion and lowered cardiac output being the two hallmarks of any Fontan circulation, it is no surprise that this includes a wide spectrum of hepatic abnormalities such as liver fibrosis, cirrhosis, or hepatocellular carcinoma (HCC), which are generally grouped and defined as Fontan-associated liver disease (FALD).4–6 Hepatic (dys)function after the Fontan operation has gained the attention of researchers and clinicians alike. However, we still lack a unified definition, a standardized flowchart for clinical surveillance, and a transparent decision-making algorithm to treat these patients. Although novel diagnostic techniques are available, some of them promising, their value in the clinical decision-making process still requires careful evaluation. This review aims to provide a practical framework for the clinical management of FALD across its entire spectrum.
Definition and Pathophysiology of FALD
Liver disease following the Fontan operation is an unwanted, but not unexpected, complication and has gained more attention in the past few years as the follow-up duration of Fontan patients has increased well into adulthood. The original study that brought this problem into the spotlight analyzed autopsy liver specimens of nine Fontan patients with chronic passive congestion seen in seven, cardiac cirrhosis in four, adenoma in one, and HCC superimposed on cirrhotic changes in one.4 Over the following years the definition of FALD has evolved, and according to recent papers, FALD encompasses a broad spectrum of hepatic congestion, severe fibrosis, and cirrhosis.7,8 It is considered to be present in every Fontan patient to some degree, but it is unclear which patients will eventually develop clinically significant advanced liver disease.5,7,9 Currently, FALD is considered a universal finding in all patients; however, according to various reports, the prevalence of high-grade fibrosis ranges from 36% to 68%.5,8,9
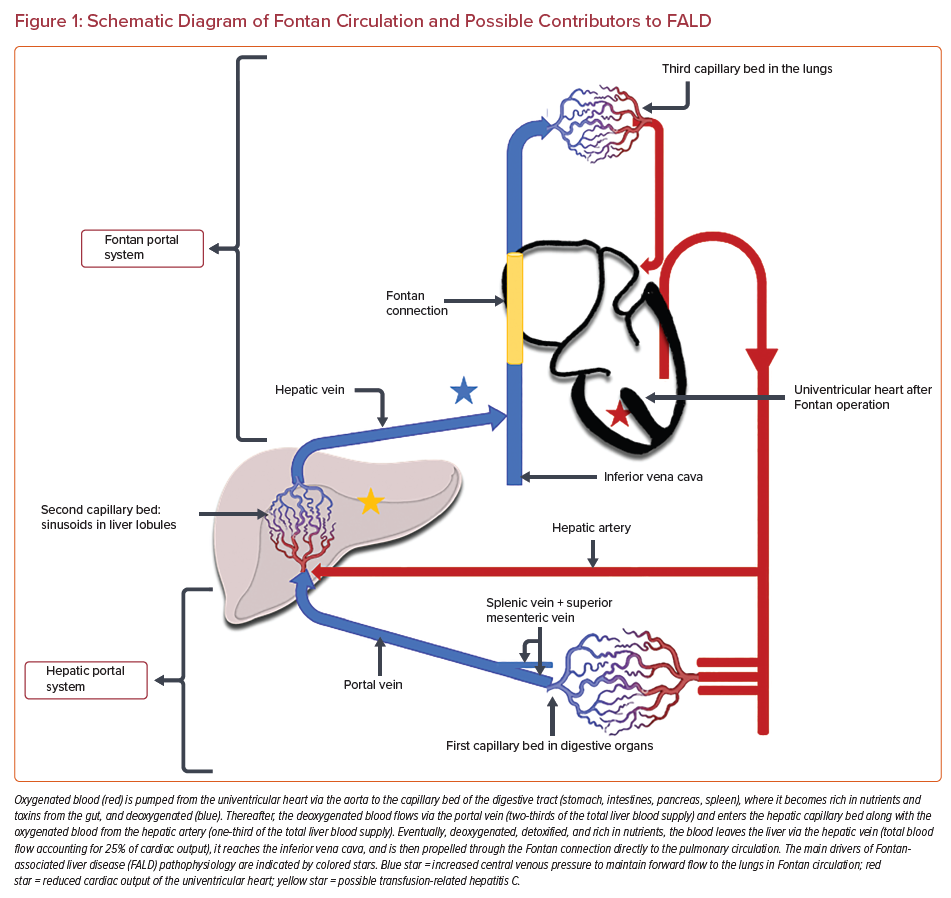
A few factors over the lifetime of Fontan patients may contribute to FALD; however, the most potent driver in this pathological pathway is a permanent hemodynamic alteration that appears after the establishment of the Fontan connection, namely elevated systemic venous pressure.4,6,10 The second contributor to the hepatic injury is probably impaired oxygen delivery caused by chronic low output and (episodes of) desaturation typical in Fontan patients.5,7,8,10,11 The combination of these two abnormalities may be particularly harmful, not only for the function of the liver but also for the intestines, with protein-losing enteropathy occurring in the Fontan patients.12 Additionally, a prenatal elevation in central venous pressure, reduced output and ischemic liver injury due to cardiogenic shock postnatally (i.e. following cardiac surgery) may also play a role in the development of FALD.5,7,8,10,11 Chronic hepatitis C infection secondary to blood transfusions may be another contributor given that many adult Fontan patients underwent the repair before the era of routine screening in blood donors (i.e. before 1992). Fortunately, after 1992 the risk of hepatitis C infection after cardiac surgery in childhood dropped from nearly 15% to less than 0.5%.13,14 Another potential mechanism for liver fibrosis has been proposed recently, suggesting the presence of ‘profibrotic milieu’ after Fontan repair. This thesis is supported by the fact that in Fontan patients the fibrotic process is diffused and affects both the heart and the liver. Fibrosis might result from the dysregulated activity of fibroblasts that impairs collagen turnover through the abnormal production and release of metalloproteinases and tissue inhibitors of metalloproteinases.15 However, it is still unknown whether any specific factor in the Fontan circulation promotes fibrogenesis. Fibrogenesis may also be multifactorial, the end result of a long-term process that routinely leads to profibrotic changes such as inflammation and cyanosis, hemodynamic derangement, venous and lymphatic congestion, or vascular trauma.2,15 A schematic diagram of the Fontan circulation focusing on the main factors of liver injury in this population is given in Figure 1.
All of the considerations regarding liver fibrosis in Fontan patients become clinically relevant as parenchymal derangements deteriorate into irreversible liver cirrhosis and portal hypertension (PH).6 In contrast to typically asymptomatic fibrosis, PH is characterized by overt signs and symptoms and is a marker of poor prognosis.2,16 A VAST score ≥2 is an indicator of PH, and the score uses typical PH features (1 point for each: lower esophageal varices [V], ascites [A], splenomegaly [S] and thrombocytopenia [V]). As demonstrated in the VAST study by Elder et al., the presence of PH (VAST score ≥2) increases the risk of adverse outcomes (death, need for a transplant, HCC) in the Fontan cohort by ninefold.16 Management includes targeted therapies (variceal band ligation or sclerotherapy, paracentesis) to reduce the clinical manifestation of PH, and early referral for the heart transplant qualification process.6
Moreover, cirrhosis is the most potent single risk factor for HCC, which in Fontan patients conveys a dramatically poor prognosis with a 1-year survival rate of 50%.17,18 According to the literature, the annual risk of HCC in the Fontan cohort with cirrhotic lesions ranges between 1.5% and 5% and is not higher when compared with patients with other causes of liver cirrhosis.10,17,19 However, the prevalence of HCC correlates with Fontan circuit duration, and its incidence is reported to increase significantly from 3% at 20 years to 13% at 30 years after surgery.4,19–21 Optimal management is not well delineated, and an individualized approach should be proposed, considering surgical resection, local radiotherapy, or trans-arterial chemoembolization.2,20 Heart–liver transplantation in the case of HCC, although theoretically possible, is at times, not a realistic option.7
Liver Histology in FALD
The specificity of the ultrastructural changes seen in FALD most probably results from the unique hemodynamic pattern created by the Fontan circulation and suggests that FALD is a congestive hepatopathy.2,20 Directly connected to the pulmonary circulation, the hepatic veins (responsible for ±25% of venous return) now function as portal vessels to the lungs (Figure 1).22 As mentioned earlier, the blood flow to the pulmonary vascular bed is passive and can be maintained only in the setting of chronic elevation of the central venous pressure. Aside from keeping systemic venous pressures low (in a normal biventricular circulation) the subpulmonary ventricle and the atrium also buffer venous return (which fluctuates with respiration) in order to keep systemic stroke volume constant at rest and during exercise.23,24 In a Fontan circulation, Fontan pressures (and hence systemic venous pressures) often increase to >25 mmHg during exercise, potentially imposing additional stress on the liver.11 Indeed, increased hepatic afterload is then transmitted to the hepatic sinusoids and proportionally to the portal vein (given that sinusoids lack valves). With portal vein flow being attenuated, hepatic artery blood flow increases through autoregulation. Nevertheless, as venous pressure further increases and exceeds 20–25 mmHg, the compensating mechanism fails, leading to reduced hepatic perfusion and subsequent liver injury. The presence of arterialized nodules often observed in liver cross-sectional imaging in Fontan patients is evidence of increased arterial blood flow.25,26
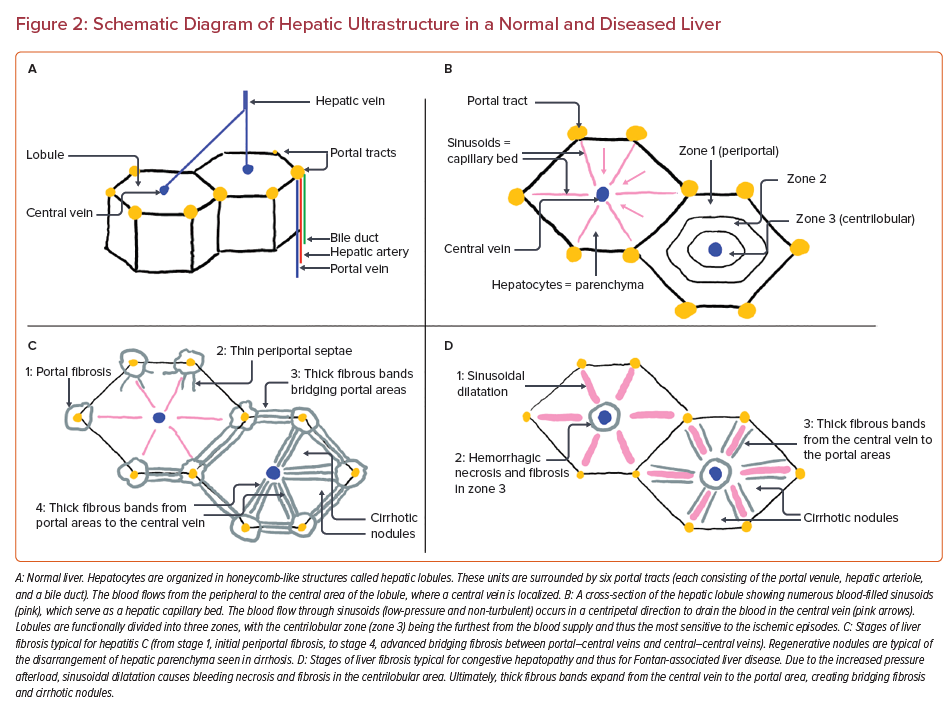
Figure 2 shows a schematic diagram of the histological structure seen in a normal liver compared with the abnormalities present in FALD. A permanently increased hepatic afterload causes liver congestion that imposes repetitive mechanical stretch on sinusoids and compression on other hepatic cells. This results in sinusoidal dilatation and activation of fibrogenesis primarily localized in the peri-sinusoidal areas.6,7 The second driver of hepatic injury is impaired hepatic oxygen delivery caused by the permanently decreased cardiac output typical in Fontan patients.5,7,8,10,11 This process promotes hemorrhagic necrosis and excessive fibrotic changes in the centrilobular localization (zone 3).10 Zone 3 is susceptible to ischemic episodes because this area is the furthest from the arterial blood supply, given that hepatic arterioles are localized peripherally, in the portal tracts.2,6,10 Further structural and functional alterations of the liver parenchyma involve bridging fibrosis linking central veins to normal portal tracts (so-called ‘reversed polarity’), in contrast to the initial periportal fibrosis encountered in liver disease due to other causes. Eventually, thick fibrous bands link the central veins, causing nodular regeneration and distorted organ architecture typical of cirrhosis (Figure 2). The pattern of fibrotic lesions in congestive hepatopathy is heterogenous with a characteristic patchy and predominantly subcapsular distribution.20,27,28
Diagnostic Work-up of FALD
FALD includes various hepatic abnormalities and is usually considered a disease continuum, starting from passive hepatic congestion through to fibrosis, cirrhosis, and HCC as end-stage organ damage. We believe that the clinical challenge lies in differentiating between mild abnormalities (which will be present in virtually every patient) and severe hepatic disease (in some patients), including cirrhotic changes or superimposed neoplasm, given that only the latter will imply a significant shift in routine clinical management. Indeed, the presence of PH associated with cirrhosis increases the risk of serious complications such as lower esophageal varices, gastrointestinal bleeding, and ascites. Ideally, our diagnostic algorithm would encompass a step-by-step approach, generating sufficient information from easy-to-implement, low-cost investigations while reserving the more time- and resource-consuming, invasive tests for selected patients.
Serum Biomarkers
A basic blood panel includes routine liver biochemistry (alanine transaminase [ALT], aspartate transaminase [AST], bilirubin, alkaline phosphatase [ALP], and γ-glutamyl transferase [GGT]), functional liver tests (international normalized ratio [INR], prothrombin time, platelets, and albumin), and hepatitis B and C screening. As reported by various authors, a progressive elevation of the serum cholestasis markers (ALP, GGT, total and direct bilirubin) with age is typical for congestive hepatopathy, including FALD.2,20,29 Still, it does not correlate with the degree of fibrosis. Indeed, in the Fontan cohort, only INR had a significant moderate correlation with high-stage fibrosis, although the usefulness of this parameter is limited due to the high rate of patients receiving vitamin K antagonists.9 To overcome this problem a MELD-XI score (Model of End-stage Liver Disease without INR; which calculates the logarithmic relationship between the serum creatinine and bilirubin) has been developed. Evans et al. noted a correlation of this parameter with the degree of fibrosis on liver biopsy in Fontan patients, but no cut-off point of sufficient specificity and sensitivity was identified.30 In contrast, Munsterman et al. analyzed various possible scores developed for liver cirrhosis patients and found that neither MELD-XI nor commercial ELF (Enhanced Liver Fibrosis Test®, Siemens) or any other specific indices had proven clinical relevance in the Fontan cohort.8,31,32 Apart from fibrosis-specific serum biomarkers, α-fetoprotein (AFP) should also be routinely checked as a marker of possible conversion of cirrhosis to HCC. Increased AFP concentration along with abnormal imaging or features of cirrhosis/PH (VAST score ≥2 points) should warrant an obligatory liver biopsy; however, the risk of needle-track seeding approaches 3%.6,19,20
Ultrasound Imaging of FALD
As part of a routine surveillance protocol, a regular liver ultrasound plays a pivotal role, especially in identifying nodules and masses in the liver. Early signs of FALD attributable to chronic congestion (hepatomegaly and dilated hepatic veins) may be encountered in almost every Fontan patient, but radiological features such as surface irregularity, coarsening of the parenchyma, focal nodular hyperplasia-like nodules, and left lobe hypertrophy indicate more advanced liver damage.10,33 Although hyperechoic lesions and irregular liver surface may be present in most patients, they are not specific for a cirrhotic process in the Fontan population.7,25 The clinical application of ultrasound elastography (FibroScanTM, Echo-Sens) is limited, given that liver stiffness measured by this modality may not necessarily result only from the fibrotic changes but may also result from parenchymal congestion.2,7,10 Following the trends over time may have clinical utility, although robust evidence is lacking.
Advanced Non-invasive Imaging of FALD
MRI or triple-phase CT (in the case of contraindications for MRI) should be implemented if progressive lesions are identified on liver ultrasound because they may better differentiate between benign and malignant processes.7,10 Typically for congestive hepatopathy, a heterogeneous mosaic or reticular enhancement of the hepatic parenchyma can be found also in FALD. Attention must be paid to the arterially enhanced nodules that may suggest HCC. However, the diagnostic process of HCC is complex, given that the hyper-enhancing nodules and vascular anomalies seen in FALD may mimic typical HCC arterially enhanced nodules.10 Fortunately, in most patients these findings correspond to non-neoplastic regenerative nodules and focal nodular hyperplasia.26,34 Nevertheless, the lesions are highly suggestive of HCC if accompanied by portal venous phase contrast washout, mosaic architecture, elevated AFP concentration, or signs of cirrhosis and/or PH. In this case, a confirmatory liver biopsy is always required.18,20,26 Promising novel tools involving T1- and T2-weighted sequences on MRI are under research, but so far they have not been found to perform better in discriminating between low- and high-grade fibrosis in Fontan patients.8,10,35 The limitations of MRI elastography in clinical practice are similar to those of the ultrasound technique. The evaluation of liver stiffness may be misleading because not only fibrosis but also congestion can contribute to it.2,7,10
Liver Biopsy
Liver biopsy results obtained in consecutive Fontan patients indicate high-grade fibrosis in 36–68% of the Fontan cohort, whereas cirrhotic lesions are present in 5–21%.5,8,36 A classification of histopathological findings remains controversial because there is no unified approach in scoring systems that would encompass all of the abnormalities seen in Fontan patients (a combination of centrilobular, peri-sinusoidal, and portal fibrosis).8,9 Nevertheless, a classification implemented in congestive hepatopathy could be used in the Fontan population (Figure 2D), for instance, a congestive heart failure fibrosis score, where 0 = no fibrosis; 1 = central zone fibrosis; 2A = central zone and mild portal fibrosis; 2B = at least moderate portal fibrosis and central zone fibrosis; 3 = bridging fibrosis; and 4 = cirrhosis.37 Sirius red staining has also been suggested to quantify the collagen deposits better, thus enabling a standardized evaluation of the fibrosis extent.5,9 Widely used scoring systems such as METAVIR or the Ishak classification (Figure 2C) are mainly dedicated to assessing fibrosis related to hepatitis, thus they are less applicable in this population.8,20,38
Even though liver biopsy remains the standard of fibrosis evaluation, the results can be biased due to subjective assessment, quality of sampling, and different types of staining.2,5,8 We usually perform a transjugular liver biopsy to simultaneously obtain a hepatic venous pressure gradient (reflecting the intrahepatic contribution to PH). Care should be taken to obtain a sufficient number of samples, taking into consideration the patchy, often subcapsular distribution of fibrosis in patients with congestive hepatopathy. Similarly, one should be aware that transhepatic gradients of more than 2–3 mmHg are rarely demonstrated, probably due to intrahepatic macro- and micro-veno-venous collateralization. Thus, a definite exclusion of PH based on these measurements is problematic. Due to the invasive character of biopsy and the (albeit small) risk of bleeding complications, alternative methods of fibrosis detection have been widely introduced into research projects assessing their credibility and feasibility. Up to now, none of these methods has been proven to accurately assess the severity of fibrosis in the Fontan cohort.2,8 Hence, liver biopsy remains the gold standard for the assessment of the severity of FALD in Fontan patients.
Practical Surveillance Model
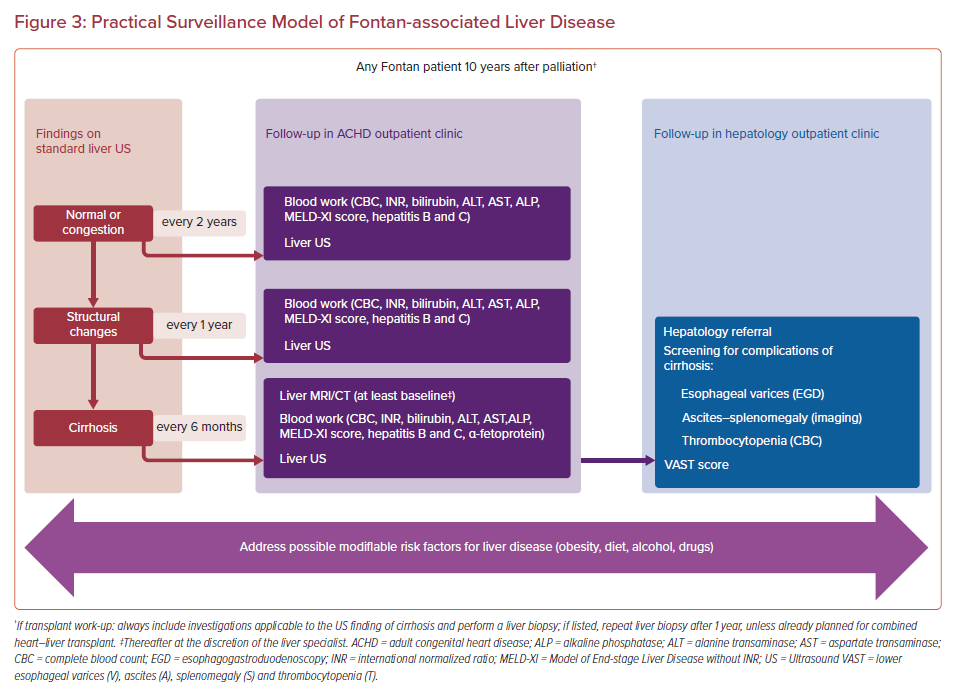
Specific surveillance strategies have been proposed to identify, follow and treat FALD, but the diversity of methods proposed and the lack of a unified algorithm remain challenging for practitioners.2,7 Undoubtedly, Fontan patients should be screened for FALD given that the extent of fibrotic changes worsens with time after the operation, along with an increase in the central venous pressure.6,36 Moreover, early identification of FALD could trigger interventions to optimize the Fontan circulation to prevent or slow down FALD progression. Finally, especially in the case of cirrhosis, HCC surveillance should be initiated. Here, we would suggest an approach to address the most clinically significant questions: first, the choice of the timing of extensive diagnostic work-up to confirm advanced fibrosis, cirrhosis, or PH that would require hepatologist consultation and specific management; and second, the reliable assessment of FALD severity and reversibility while listing the patient for the heart transplant or heart–liver transplant.
The flowchart of FALD surveillance in adults after the Fontan operation is shown in Figure 3. Starting at 10 years after surgery, blood work (AST, ALT, GGT, bilirubin, ALP, albumin, INR, complete blood count, MELD-XI, hepatitis B and C) and ultrasound imaging should be performed at least every 2 years.2,10,20 In case of structural changes seen on the liver ultrasound, the follow-up period should shorten to 1 year. Subsequently, the diagnosis of cirrhosis should prompt an ultrasound imaging and blood work performed every 6 months. Additional imaging studies (MRI liver, triple-phase CT, Fibroscan) and a hepatologist consultation should be scheduled. In our practice, we currently do not perform elastography. A qualification protocol for transplant listing should encompass comprehensive blood work, liver ultrasound, MRI liver, liver biopsy, and hepatology consultation. Referring to hepatologists is an essential step in terms of shared decision-making but it should be stressed that this cannot lead to diffusion of responsibilities because the main driver of FALD remains Fontan circulatory failure.
Heart versus Heart–Liver Transplant in FALD
The listing for an orthotopic heart transplant (OHT) versus combined heart–liver transplant (CHLT) is an ultimate therapeutic choice and nowadays is gaining more attention.2,7 In the context of FALD, a fundamental question when discussing heart transplant eligibility is related to the reversibility of liver damage, the added perioperative transplant risk related to reduced liver function, the risk of post-transplant progression of liver disease and the risk of HCC. According to the UCLA Adult Congenital Heart Disease Center, the criterion of irreversibility of liver disease in Fontan patients would be met in the case of cirrhosis or advanced (bridging) fibrosis on liver biopsy. Other findings attributable to advanced organ failure, that is, lower esophageal varices, ascites, splenomegaly and thrombocytopenia, suggestive of PH, would also increase the patient’s eligibility for a heart–liver transplant.39 In those patients, CHLT should be considered because the short-term outcomes are similar to heart-only transplants (a 1-year survival after CHLT in 86–100% of patients).7,39–41 Additionally, the combined procedure reduces the risk of cardiac allograft rejection because the transplantation of the liver before the heart may facilitate the absorption of donor-specific antibodies.6 After CHLT, lower immunosuppression levels are required, probably due to the induction of partial tolerance, which permits acceptance of other simultaneously transplanted organs.42
Unfortunately, in the real-life setting, it is possible that listing the Fontan patient for heart–liver transplant would significantly decrease the patient’s chances of either surviving until they are added to the transplant list or, when listed, surviving until the transplant. Therefore, some expert centers advocate for OHT even in the case of advanced FALD because bridging fibrosis (stage 3 in the METAVIR score) in congestive hepatopathy is known for its reversibility after resolution of the offending injury.6,28,43 In a case series by Broda et al. there was no hepatic disease progression after heart-only transplant on mid- and long-term follow-up. In 8 out of 10 patients, pre-transplant liver cirrhosis was recognized on imaging, although no confirmatory liver biopsies were performed.44 In a multi-center study involving 109 pediatric Fontan patients, pre-transplant FALD (although recognized in the vast majority of patients) was not a significant cause of early mortality.45 In our opinion, a decision on eligibility for the CHLT waitlist should be treated as a last resort: only in the case of extensive cirrhosis on liver biopsy, PH, or end-stage liver failure. In Fontan patients listed for heart transplantation, (advanced) fibrosis should raise the possibility of a heart–liver transplant, but may not be the deciding factor to consider CHLT.
Pregnancy in FALD
According to the current guidelines, women with any complications after a Fontan operation should be counseled against pregnancy.46,47 However, there is emerging data that in clinically stable patients with good ventricular function and no episodes of atrial tachyarrhythmia or thromboembolism, pregnancy should be well tolerated.48 Aside from maternal complications in Fontan patients such as arrhythmia and heart failure, miscarriage, prematurity, or intrauterine growth restriction remain the main obstetric issues. An individualized approach in pre-conception considerations should also be used in the context of FALD because the risk is undoubtedly higher in patients with hepatic fibrosis or cirrhosis than in those with mild liver disease.49 According to the Chinese study on pregnant women with concomitant cirrhosis, severe maternal complications including placenta abruption, postpartum hemorrhage >1,000 ml, hysterectomy, multiorgan failure, and upper gastrointestinal bleeding were present in as many as 32% of cases. Also, conception itself is uncommon in the setting of cirrhosis because of endocrinal disturbances, particularly in estrogen metabolism.50
Pre-conception counseling of Fontan patients should consist of laboratory evaluation of the hepatic function and ultrasound of the liver and spleen. In the case of thrombocytopenia, coagulopathy, elevated aminotransferases, hyperbilirubinemia, splenomegaly, or liver mass, hepatologist consultation is warranted.51 The biggest concern during pregnancy is related rather to the presence of PH and varices than to synthetic dysfunction. There is a low threshold for esophagogastroduodenoscopy (EGD) to detect varices. If present, the management options include pharmacotherapy with non-selective β-blockers throughout the pregnancy or invasive treatment in the case of advanced-stage disease (preferably during the second trimester).49 Importantly, pregnant women with Fontan circulation have the highest risk of antenatal and peripartum bleeding in patients with congenital heart disease, and the overlapping FALD-associated coagulopathy may exacerbate this complication.50,52
Responsibility of the Cardiologist Towards the Liver
It should be highlighted that the leading cause of FALD is abnormal circulation and that suboptimal hemodynamics will accelerate liver changes. Therefore, FALD cannot be seen separately from the Fontan procedure and Fontan hemodynamics. This process starts early in childhood. Indeed, when creating the Fontan circulation, hence rendering systemic venous return into portal vessels to the lungs, a low pulmonary vascular resistance is of paramount importance. The pediatric cardiologist and congenital cardiac surgeon should carefully plan the staged construction of Fontan circulation, understanding that the pulmonary vascular bed will be the most limiting factor in this circuit.1,22 A low pulmonary vascular resistance is more likely if sufficient growth of pulmonary arteries prior to referral for a Glenn procedure is guaranteed. Later in life it is crucial to optimize the hemodynamics by decreasing any potential resistance in pre-pulmonary and pulmonary vessels. This could be achieved by means of interventional procedures such as stent expansion in small conduits or stenting the hypoplastic or narrowed pulmonary arteries.22 Diuretics could reduce liver congestion at the expense of lower cardiac output. Hence, there is an urgent need for innovative solutions, such as the introduction of a subpulmonary assist device that could also alleviate liver congestion. The attempts to facilitate the drainage of central venous congestion with artificial devices are promising but very limited.53