Mitral regurgitation (MR) is the most common valvular heart disease throughout the world, present in approximately 10% of people aged ≥75 years, with an estimated case prevalence of 4 million in the US alone.1,2 Moderate to severe MR is associated with significantly high mortality, which can be as high as 20% at 1 year and 50% at 5 years.3–5 Although surgical mitral valve repair or replacement remains the standard of care in severe primary MR, several patients with MR can now be treated by transcatheter percutaneous edge-to-edge mitral valve repair using the MitraClip (Abbott).6,7 Transcatheter mitral valve repair with MitraClip was initially approved by the Food and Drug Administration in 2013 for patients with prohibitive surgical risk in the setting of primary MR. Subsequently, in 2019, this therapy was also approved for patients with secondary MR who remain symptomatic with heart failure despite optimal medical therapy. Since then, the use of MitraClip has rapidly expanded, and currently more complex anatomies are being treated, with progressive improvement in peri-procedural clinical outcomes.8 The 3-year follow-up data from the COAPT trial show that in such patients with heart failure and moderate-severe secondary MR who remain symptomatic despite guideline-directed medical therapy (GDMT), mitral valve transcatheter edge to edge repair (TEER) is a safe procedure that improves survival, quality of life and functional capacity while reducing the rate of heart failure hospitalizations compared to GDMT alone.9
The primary focus of transcutaneous mitral valve repair with the MitraClip is to reduce the severity of MR by permanently apposing mitral leaflets.10,11 This complex procedure requires an in-depth understanding of mitral valve anatomy and mastery of maneuvering technical skills to achieve optimal success results with minimal procedural complications. This review article will discuss advanced steering maneuvers to achieve optimal success in MitraClip procedure for management of symptomatic patients with MR in some common, challenging clinical situations.
Eligibility Criteria
For safe and successful positioning of the clip, several anatomical eligibility criteria have been established. According to initial randomized clinical trials, a coaptation gap of >2 mm, a coaptation length of <11 mm in functional MR and in degenerative mitral valves, a flail gap of <10 mm, and a flail width of <15 mm are considered favorable for procedure success rate.12 Most patients included in the earlier trials had central A2–P2 scallop pathologies.
The MitraClip System
The MitraClip is a cobalt chromium, polyester-covered device with movable arms. It is a transvenous transfemoral device implanted via a 24 Fr steerable guiding catheter (SGC). The MitraClip delivery system has two main steering components: a steerable guiding catheter (SGC) and a clip delivery system (CDS). The clip delivery system consists of steerable sleeve (SS) and a delivery catheter (DC). The SGC has an 80 cm working length with a maximum outer diameter of 24 Fr. The SGC has a mounted knob (+/−) which allows flexing (+) and straightening (−) of the catheter tip. In the current MitraClip system, the CDS key material has been changed from stainless steel to nylon, which has improved advancement and straddling. Furthermore, the plane of curvature of the SS has been modified in the MitraClip NT system to improve alignment between the SS and SGC. The MitraClip NT system has gripper material made of Elgiloy instead of Nitinol, which allows an increased gripper drop angle that facilitates the leaflet capture. The MitraClip system has undergone several updates, with the current clips available in wider and longer arm lengths (NT, NTW, XT and XTW) in most of the implanting institutions. The NT MitraClip is appropriate for smaller leaflets, restricted mitral leaflets, secondary MR without a wide coaptation gap, focal leaflet pathologies, smaller valve areas and patients with a higher starting mitral valve gradient. The wider clips (NTW and XTW) can be used for patients with wide flail or prolapsing segments with adequate starting valve area/gradients. The XT MitraClips are most suitable for patients with a larger coaptation gaps as well as large prolapses or flails. Supplementary Table 1 provides a summary of the dimensions of the available MitraClip sizes.
Transseptal Puncture
Transseptal puncture is one of the most important initial steps of the MitraClip procedure. The optimal puncture site for the MitraClip procedure is located mid-superior (along the inferior-superior axis in the bicaval transesophageal echocardiogram [TEE] view) and posteriorly in the interatrial septum for an ideal height of 4.5–5.0 cm above the mitral annulus in primary (degenerative) pathologies and slightly lower in secondary (functional) MR. A higher transseptal puncture height might be required for more medial MR jets. With newer generation MitraClip systems, it is always better to have more rather than less height, and thus a transseptal puncture height of ≥4 cm is usually favorable. TEE has a vital role in guiding transeptal puncture. Three TEE planes are helpful to determine the correct interatrial puncture site: a short axis view for anterior-posterior orientation, a bicaval view for superior and inferior orientation, and a four-chamber view to determine height above the mitral valve. Newer radiofrequency transseptal puncture systems have improved the safety and precision of transseptal puncture.
MitraClip Procedure
After optimal transseptal puncture, the steerable guide catheter with dilator is advanced gently into the right atrium in straightened position (using the '−' knob) and then gently advanced into left atrium at least 2–3 cm in a neutral position by placing the +/− knob to neutral. SGC with dilator is advanced into left atrium over a stiff wire (either an Amplatz wire parked in the left superior vein or a pre-looped wire like Inoue or Baylis ProTrak wire in the left atrium) under fluoroscopic and TEE guidance. The position of the dilator can be easily seen as a cone-shaped ring and an echogenic ridge on the catheter system. Advancement of the SGC should be done gently and always under imaging guidance to avoid injuries to free left-atrial wall. After the SGC is placed safely in the left atrium, the wire and dilator are retrieved while maintaining continuous suction to avoid air embolism. Once the SGC has been placed, the CDS is advanced via SGC under fluoroscopy and TEE guidance towards the left upper pulmonary vein. As the CDS exits the sheath, it is necessary that markers in the system straddle the sheath marker before the clip is moved towards the mitral valve.
Straddling should be performed gently under fluoroscopic and transesophageal guidance to avoid injuries to left atrial wall and surrounding structures. Once straddled into the left atrium, the medial (M knob) and slight posterior torque (by clockwise rotation of the SGC) with retraction of the entire system brings the clip closer to the mitral valve. In cases where the left atrium is smaller size, the SGC can be torqued counterclockwise to move anteriorly, followed by use of the '+' knob to gain extra room for straddling.
The DC handle is used to perform advancement and retraction of the MitraClip for grasping and alignment of MitraClip arms perpendicular to the coaptation plane of the mitral valve. As torque transmission from the DC handle to the clip arms is not 1:1, the DC handle must be advanced and retracted during rotations to maximize the rotation of clip arms and remove stored tension.
All manipulations and alignment of the clip should be performed in the left atrium above the mitral valve leaflets to avoid injuries to mitral valve apparatus, which can potentially worsen MR.13 A 3D en face ‘surgeon’s’ view on TEE allows orientation of clip arms. By torquing the DC handle clock or counterclockwise, the clip arms move perpendicular to the mitral valve coaptation line. Medial-lateral clip movements are monitored in 3D en face view and anterior-posterior movements seen in an orthogonal mid-esophageal long-axis (left ventricular outflow tract [LVOT]) view. Before advancing the clip through the mitral valve, the grippers are raised, and appropriate functioning of the grippers can be verified. Note should be made regarding which gripper the tactile marker represents in case one needs to do independent grasping of the mitral valve leaflets.
Although variable across operators, our practice has been to advance the clip in a semi-closed position (sometimes with a breath-holding maneuver, especially when placing a second adjacent clip) to avoid rotation related to translation.14 Once the clip is advanced into the left ventricle, the clip position needs to be re-examined under TEE and fluoroscopy to ensure that the clip has not rotated during the advancement into the left ventricle.Once the MitraClip has been placed in a satisfactory position, leaflets are appropriately secured between the grippers and clip arms in LVOT view. After grasping the leaflets, the adequate tissue bridge must be confirmed in multiple views. Significant motion of any leaflet might indicate poor tissue bridge from the clip and, thus, the need for re-grasping. The clip should be closed slowly, and the clip delivery system can be advanced slightly to relieve any tension built up in the system during the grasping process. Before and after the implantation of clip, assessment of stenosis and regurgitation of the mitral valve along with morphological results of the clip must be performed with echocardiographic images.15 The new MitraClip G4 system allows for individual grasping of the mitral valve leaflets.
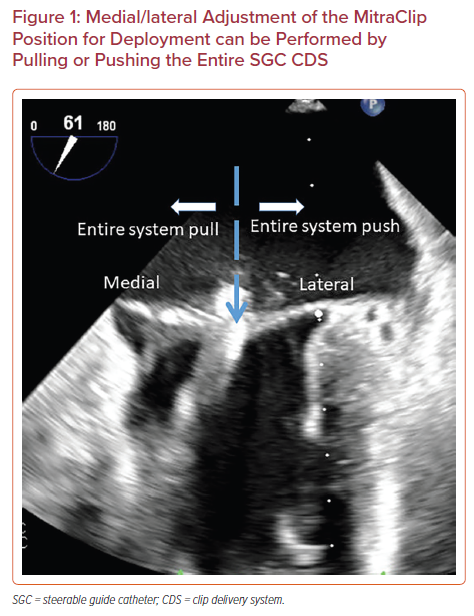
MitraClip Device Steering Maneuvers
The MitraClip system is steered with manipulations of the SGC including rotation, advancement, and use of the +/− knob and by turning of two knobs mounted on the CDS including the M and A/P knobs. Advancement of SGC (‘pushing in’) will move the clip laterally and retraction of SGC (‘pulling out’) will move the clip medially (Figure 1). Clockwise rotation of the SGC moves the clip posteriorly and counterclockwise rotation moves the clip anteriorly. The +/− knob on the SGC is commonly kept in the neutral position, unless needed for advanced maneuvers. The '+' knob moves the catheter base posteriorly to help with perpendicularity (‘standing up’), as discussed below (Figure 2).
The CDS has two mounted knobs including the M/L and the A/P knob. Both knobs perform optimally when the SGC and CDS are in the straddled position. These two knobs control three cables that run through the length of the SS and interact with each other.
The M knob moves the clip medially and anteriorly with aim to bring it perpendicular to mitral annular plane. The M knob is turned towards M until the SS is approximately 90 degrees. These turns should be performed in a stepwise fashion with slight posterior (clockwise) rotation of the SGC as SS will move anteriorly. At the same time, it is important to monitor and maintain an optimal straddling position during the M knob movement as un-straddled position will create excessive tension on the cable, which can lead to permanent set shaft. The A/P knob is usually reserved for scenarios that require advanced steering maneuvers. The A/P knob moves in two directions. Turning of the A/P knob towards A leads to movements of the SS anteriorly, and laterally, superior to the annular plane. Contrarily, turning towards P results in medial and posterior movement of SS, moving the clip down towards the valve. Supplementary Material Table 2 provides a summary of MitraClip knob functions, maneuvers, and counter-maneuvers.
Some clinical scenarios need advanced steering maneuvering to achieve successful results in management of MR with MitraClip system, which are described in the following sections.
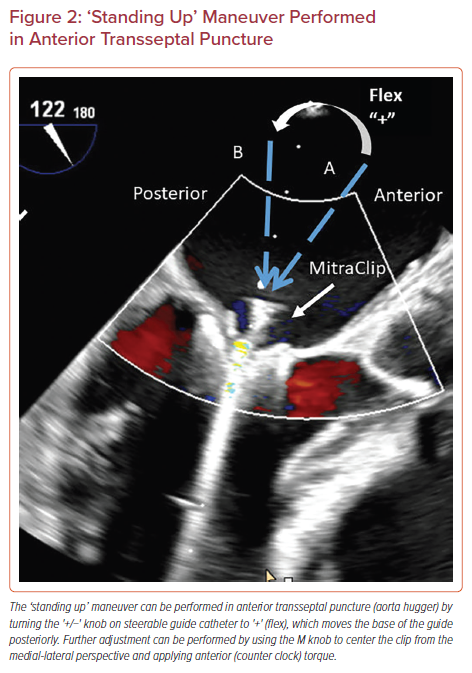
Anterior Transseptal Puncture or Aorta Hugger
As discussed earlier, posterior transseptal puncture ensures adequate height from the mitral valve. An anterior transeptal puncture will lead to considerable difficulty in leaflet grasping because of the anterior–posterior trajectory of the SS. The best solution in case of an accidental anterior puncture (due to needle slippage, etc.) is to repeat the transseptal puncture. However, if this is not an option due to anatomical reasons, then an advanced steering technique can overcome this situation.
The +/− knob on SGC is turned to + to flex the SGC, commonly known as ‘standing up’ (Figure 2). This steering maneuver moves the base of the guide posteriorly (away from the aorta), which is corrected by using the M knob to center the clip from medial-lateral perspective and anterior (counter-clock) torque of the guide.
Low Transseptal Puncture or Unfavorable Transseptal Height to Mitral Valve
The optimal site of transseptal puncture is different for degenerative (primary) MR and functional (secondary) MR. In degenerative MR, the transseptal puncture site should be 4–5 cm above the mitral annular plane to gain enough space for adequate maneuvering of the catheter and MitraClip system. In functional MR, the transseptal puncture site can be lower as the line of coaptation is usually below the mitral annulus plane due to extensive tethering. An inferior or relatively anterior transseptal puncture results in the clip being too close to the mitral annular plane, which makes retraction of the DC into left atrium limited. This leads to difficulty in arm orientation and leaflets grasping. To gain height, the SGC should be rotated clockwise (posteriorly), with anterior correction of the SS by moving the A/P knob towards A. This maneuver will move the clip anteriorly and any associated lateral movement of the clip should be corrected by using the M knob.
High Transseptal Puncture or Unfavorable Transseptal Height to Mitral Valve
High transseptal punctures are rarely a problem, especially with newer systems, which have a longer working length of CDS. However, if you need to lose height, the SGC can be rotated anteriorly (counterclockwise), while steering the CDS posterior to the mitral valve by moving P with the A/P knob. Any associated medial movement of the clip should be corrected by advancing the SGC or releasing of the M knob.
Contrarily, the M knob can be released first to move the clip laterally and posteriorly followed by the anterior torque of the guide and using the P knob to shed height as well.
Large Gap Height
The MitraClip NT spans 20 mm at fully open and 17 mm in the grasping position at 120 degrees, while the longer MitraClip XT has an additional 3 mm for each arm. The longer arms help overcome some of the challenges in grasping large flail gaps. Additional maneuvers to overcome this large gap height include rapid ventricular pacing (140–180 BPM) to create ventricular standstill or the use of adenosine.
Furthermore, adjusting the positive end-expiratory pressure and tidal volume as well as breath hold can help optimize the coaptation gap for better grasping of the valve leaflets.
MitraClip in Patients with Mitral Regurgitation Due to Dehiscence of Prior Mitral Valve
Annuloplasty Ring
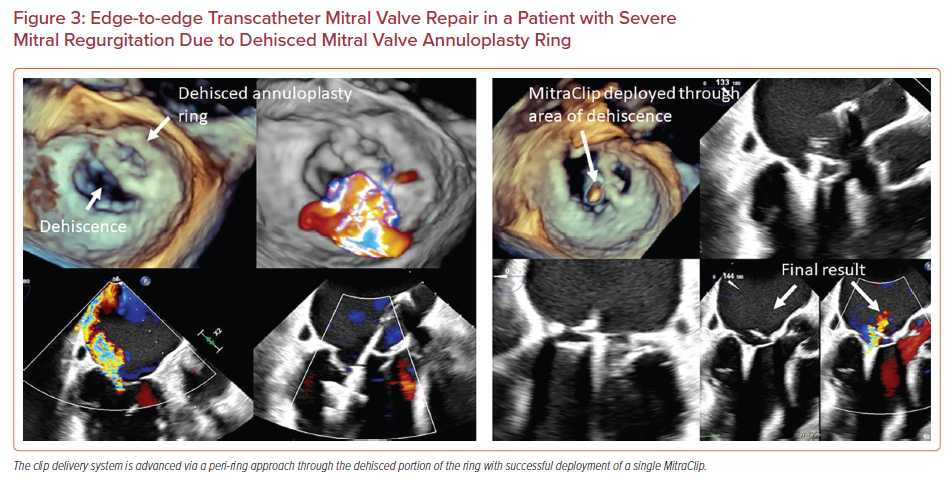
Dehiscence of the surgical mitral annuloplasty ring with associated MR is associated with poor outcomes.16–19 While some patients can undergo redo open heart surgery, others have a prohibitive surgical risk. In these patients, transcatheter edge-to-edge mitral valve repair can be performed, although the procedure requires technically challenging maneuvering.16–19 A common location for the annuloplasty ring dehiscence is the posterior annulus which is superior to the coaptation plane. In this scenario, an aorta hugger technique can be used to avoid the surgical prosthesis. This involves creating a transseptal puncture anterior to the fossa ovalis before maneuvering the CDS through the annuloplasty ring, although this might limit the clip height and subsequent maneuverability. In another approach, described by Joseph et al., transseptal puncture is performed at the regular posterior and mid-superior location.16 The '−' knob is then applied moving the clip anterior and lateral which is corrected by posterior guide torque and applying the M knob, respectively. Figure 3 shows successful deployment of a MitraClip in a patient with an annuloplasty ring that was dehisced from the lateral and posterior wall. The pathology was approached from outside the ring (posterior and lateral to the ring), with a medial trajectory created by using the M knob.
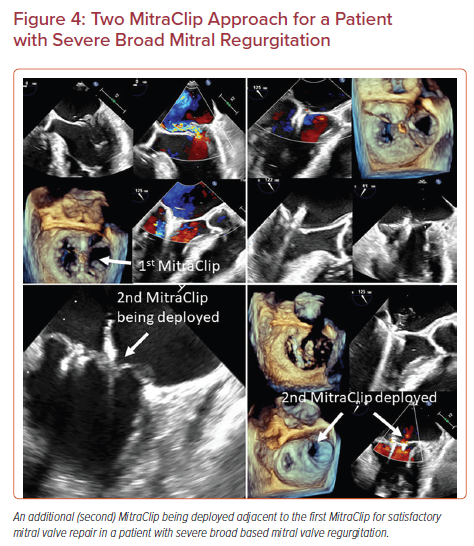
Placing Adjacent MitraClips
Selected patients with severe MR, such as those with a very broad jet of MR or a large prolapse/flail width may need a second MitraClip.20,21 In most cases, the need for a two-clip approach can be predicted based on anatomical characteristics of the mitral valve determined by pre-procedural imaging. In others, such an approach may become necessary during the procedure if a single central clip is not sufficient to significantly reduce the severity of MR.20 A two-clip approach requires special maneuvering techniques. It is easier to deploy the second clip lateral to the initial clip as the maneuvering is more straightforward. It is also easier to deploy the second clip near the central A2-P2 segments rather than in the commissures. Thus, it is recommended to plan the first clip accordingly to allow space and maneuverability for the additional clip. The second clip should be advanced into the left ventricle from the left atrium in either a close or semi-closed position (not more than 60 degrees open) to avoid interaction with the previous clip (Figure 4). Breath hold might be needed while advancing the clip in some cases to avoid the interaction with the previously placed clip. It is desirable to have the second clip positioned as close to the first one to minimize the residual mitral valve gradient.
In difficult cases where the mitral valve leaflets are difficult to grab with the MitraClip, transient asystole can be induced using adenosine injection or rapid pacing with temporary interruption of mechanical ventilation to allow ideal position of the MitraClip.22–24 In addition, ventilatory maneuvers can be attempted, which can help place the mitral leaflets in a desirable position for the deployment of the clip. For instance, during inspiration and expiration there is a respective lateral or medial shift of the heart that can affect the mitral valve leaflet position relative to the MitraClip.25 Manual ventilation can allow fine adjustment in the position of the mitral valve leaflets for optimal grasping and deployment of the MitraClip. Similarly, such movements can be minimized by holding the breath through the ventilator or reducing the tidal volume while increasing the frequency of breaths delivered.25
Redo Edge-to-edge Mitral Valve Repair
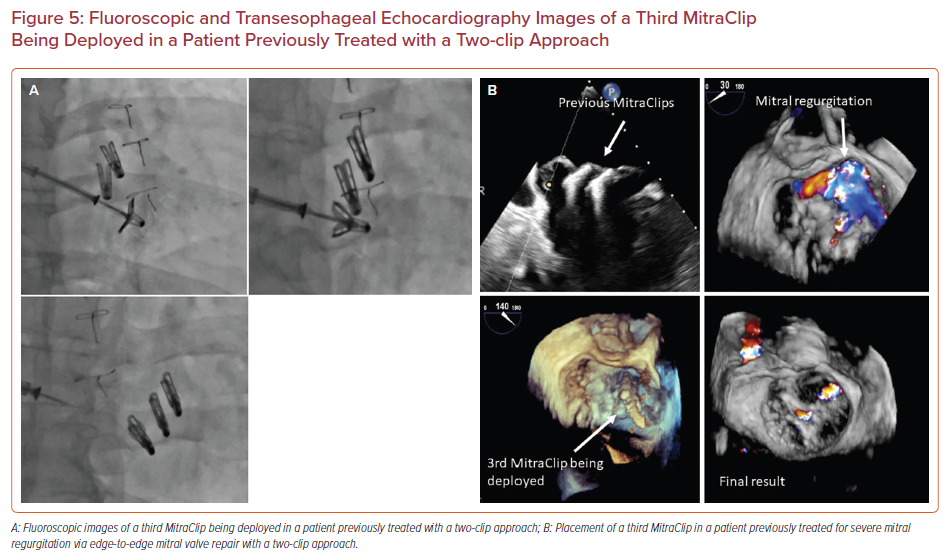
Redo edge-to-edge mitral valve repair with placement of additional MitraClip(s) is a feasible option in patients with recurrence of MR after an initial edge-to-edge mitral valve repair. Figures 5a and 5b demonstrate successful placement of a third MitraClip in a patient who had previously undergone an edge-to-edge mitral valve repair with two clips. Redo repair can be performed as long as there is no significant residual mitral valve gradient (if mean trans-mitral gradient is <5 mmHg) and the residual native valve area is >4 cm2, in the presence of a suitable valve anatomy for placement of additional MitraClip(s).26 As in the case of a two-clip approach, transient asystole with adenosine and/or rapid pacing as well as advanced ventilatory maneuvers can be used to optimize clip position and allow a better grasp of the mitral valve leaflets with the MitraClip.
Additional Scenarios Needing Special Considerations
Some clinical scenarios may require additional care and other procedures for procedural success. In patients with residual significant MR between two prior clips, a third clip can be placed in the same manner as described above for redo edge-to-edge mitral valve transcatheter repair. Care must be taken while treating commissural pathologies, such as A3/P3 prolapse, due to the risk of clip entanglement with the chords. If the anatomy is permissive and the decision is made to proceed with clip placement in the commissural area, it might be reasonable to use to smaller NT clip instead of the wide or larger XT clips. In selected situations, operators have used an Amplatzer Vascular Plug (Abbott) or CARDIOFORM occluder device (Gore Medical) for treating residual leaks in between the clips as well as in commissural residual leaks.27,28 In patients with extensive leaflet calcification, care must be taken to avoid areas with heavy calcification to prevent injury to the mitral valve leaflets during grasping.
Conclusion
Transcatheter mitral valve repair using the MitraClip is currently an effective treatment option for the management of patients with severe MR. Given the complexity of the procedure, additional unique skills in steering maneuvers of the MitraClip delivery system are essential to optimize patient outcomes. Knowledge and mastery of such advanced steering maneuvering techniques can allow operators to complete the procedure successfully with low rates of complications, even in difficult scenarios.