Coronary artery disease (CAD) affects more than 20.1 million adults in the US and remains the leading cause of mortality and morbidity.1 Acute coronary syndrome (ACS) encompasses unstable angina, ST-elevation MI (STEMI), and non-ST elevation ACS (NSTE ACS).2,3 The diagnosis of ACS is made based on clinical presentation, physical examination, ECG findings, and cardiac biomarker elevation. Early intervention for those with ACS can be life-saving; thus, appropriate risk stratification is crucial.
Atherosclerotic lesions develop over many years, with one of the longest incubation periods known for human diseases.4 The pathophysiology of ACS involves disruption of coronary atherosclerotic plaque, leading to activation of platelets and the coagulation cascade. Plaque rupture can subsequently lead to coronary artery occlusion, often without warning or with angina.4–7 Angina pectoris is defined as chest discomfort attributed to myocardial ischemia. The Canadian Cardiovascular Society angina classification is a symptom severity scale used to assess and grade physical limitation due to symptoms.8,9 Studies have shown greater angina severity at the time of diagnosis to be associated with higher mortality rates, cardiovascular hospitalizations, coronary revascularization, and overall healthcare costs.10–12
The most common clinical presentation of ACS is acute chest pain; however, triaging and identifying the underlying cause of chest pain can be a diagnostically challenging feat because most individuals with acute chest pain do not have an ACS. Indeed, chest pain is the second-most common presenting concern of patients in emergency departments (EDs) in the US, representing approximately 6.5 million ED visits annually.13 However, only 5.1% of patients presenting to the ED with chest pain are ultimately diagnosed with ACS, whereas more than half of these visits for chest pain are attributable to non-cardiac causes.14
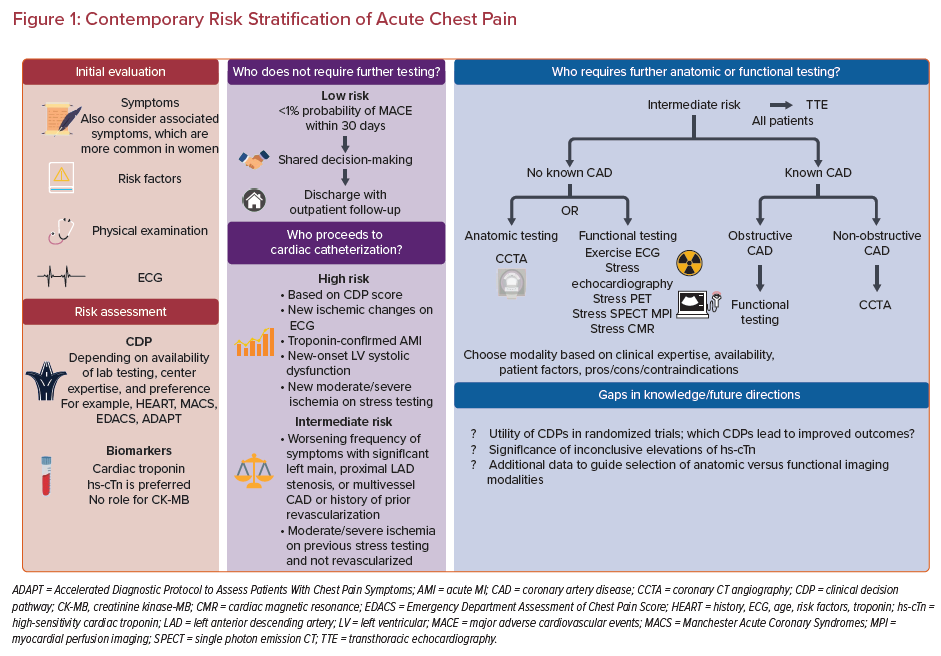
The 2021 American Heart Association (AHA)/American College of Cardiology (ACC) guidelines for the evaluation and diagnosis of chest pain represent the first-ever guidelines for the evaluation of patients with acute chest pain and offer recommendations for risk stratification in this population.15 In this review, we summarize contemporary risk stratification methods for ACS as recommended by these guidelines (Figure 1).
Role of Clinical Decision Pathways
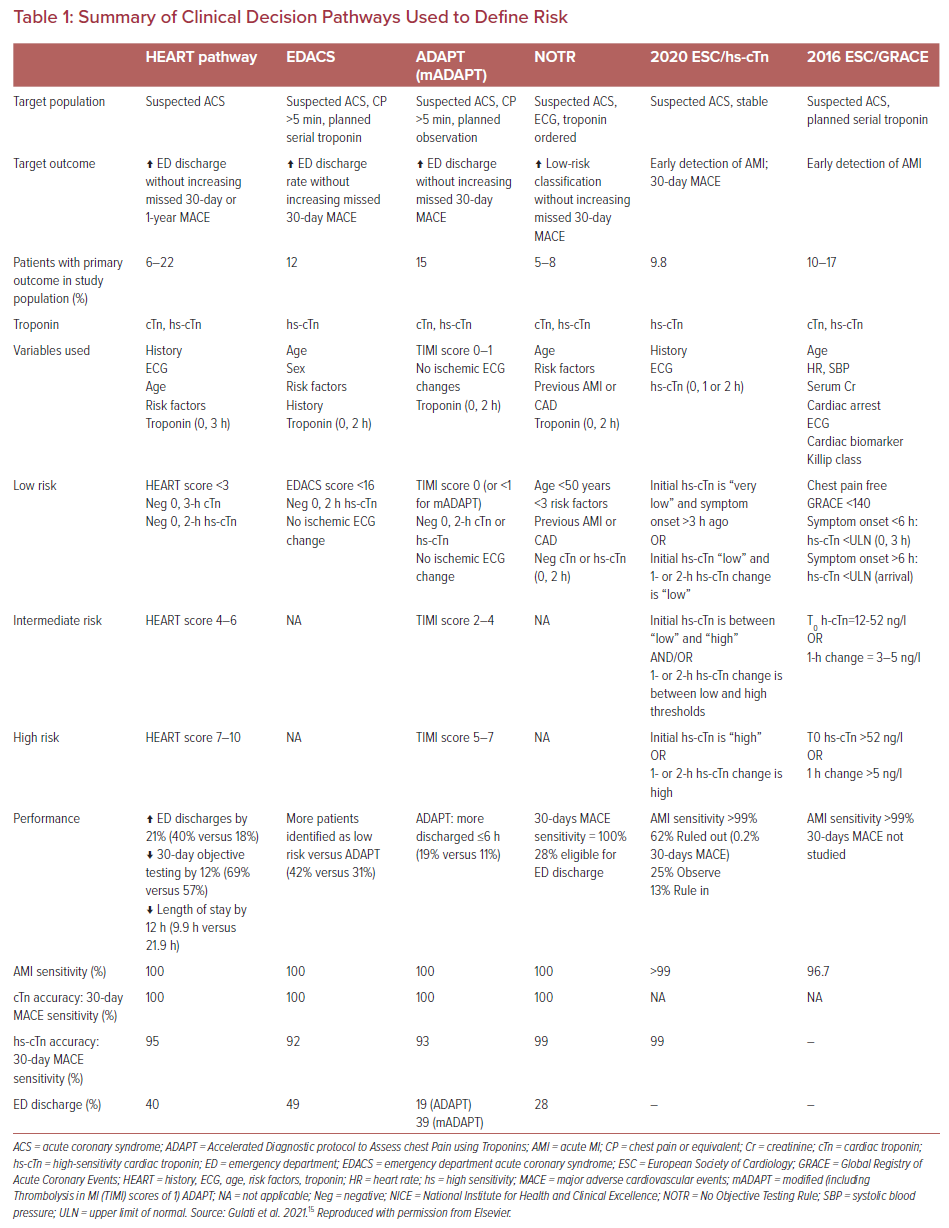
Multiple clinical decision pathways (CDPs) have been proposed over the past decade to guide the diagnosis and management of ACS. The history, ECG, age, risk factors, troponin (HEART) score, Vancouver Chest Pain Rule, Manchester Acute Coronary Syndromes (MACS) rule, Emergency Department Assessment of Chest Pain Score (EDACS), and Accelerated Diagnostic Protocol to Assess Patients With Chest Pain Symptoms (ADAPT) are commonly known decision rules that incorporate scoring systems that are used in the ED to risk stratify patients presenting with chest pain based on the probability of having ACS and the risk of 30-day major adverse cardiovascular events (MACE).16-19
Such decision rules have been incorporated into CDPs helping to categorize patients into different risk classifications, aiding clinicians in making subsequent diagnostic and disposition plans. Using a CDP helps avoid unnecessary testing and reduce admissions in low-risk patients while maintaining sufficient specificity to identify individuals who are at higher risk for MACE and require additional testing. The use of these CDPs varies from center to center based on the type of troponin assay available, center expertise, risk tolerance, and preference for one or another specific pathway. The 2021 AHA/ACC guidelines do not endorse one specific CDP, but rather advise the use of CDPs based on each center’s availability and expertise.15 CDPs should be implemented at the institutional level, with multidisciplinary teams, to standardize and ensure consistency in decision making.15
A summary of such risk score-based CDPs is provided in Table 1. Overall, comparative studies have shown that the HEART score outperforms other risk scores, such as the Global Registry in Acute Coronary Events (GRACE) and Thrombolysis in MI (TIMI) risk scores, in terms of predicting MACE.20 The original HEART score is limited because it is based on a single measurement of older-generation troponin assay. The HEART pathway overcame this limitation by using serial troponins.21,22 EDACS and Troponin-only Manchester Acute Coronary Syndromes (T-MACS) have also shown comparable performance with the HEART score.22–25 All these scores show high sensitivity and negative predictive value at the cost of specificity and positive predictive value for their outcome.26 It should be noted that because sex-specific considerations are not included in all scoring systems, their effectiveness in men and women may be different.27 Despite the numerous risk stratification scores and various comparative studies, it is still unclear which risk score or pathway is considered best in assessing patients presenting with chest pain in the ED. Hence, most ED clinicians opt to use the risk assessment tool based on institutional experience and comfort with the scoring system. Nevertheless, it is noteworthy that all the scoring systems are to assist rather than dictate decisions, and should therefore be used alongside clinical judgment.
Role of Biomarkers
Cardiac biomarkers are valuable tools for the diagnosis of and risk stratification for ACS. ACS most commonly occurs due to rupture or erosion of an unstable atherosclerotic plaque, leading to acute thrombotic occlusion of the coronary artery. The downstream myocyte necrosis and associated cell membrane disruption lead to the release of intracellular proteins that can be quantified in the blood. Several markers have historically been used to detect myocardial injury, including aspartate transaminase, lactate dehydrogenase, myoglobin, creatinine kinase (CK), and CK-MB.28-31 However, these previously used biomarkers were limited due to their lack of specificity for cardiac tissue, and in the current era cardiac troponin (cTn) I or T, preferably using high-sensitivity assays (hs-cTn), is broadly recommended.2 In fact, the 2021 AHA/ACC chest pain guidelines provide a class I recommendation for the use of hs-cTn and a class III recommendation against the use of CK-MB for the diagnosis of acute myocardial injury due to a lack of specificity.15
In the past few decades, cTn has emerged as the biomarker of choice due to its sensitivity and specificity for cardiac damage. Detection of a rise or fall in cTn concentrations, drawn 1–3 h apart for hs-cTn or 3–6 h apart for conventional cTn, is used to diagnose acute MI, with “increased cTn” being defined as a value exceeding the 99th percentile of the normal reference range for the specific assay.2,32 The rising and/or falling pattern is essential to distinguish acute cTn elevations from chronic elevations, which can occur in patients with comorbidities such as heart failure or chronic kidney disease.33 Elevated cTn concentrations are strongly associated with an increased risk of index and recurrent cardiovascular events.34 The relationship between baseline cTn and outcomes in patients with NSTE ACS has been well established, with higher cTn concentrations being associated with significantly higher 30-day mortality in the GUSTO-IIa troponin T substudy.34,35 In addition, in a secondary analysis of the TRILOGY ACS trial, comprising patients who were medically managed for NSTE ACS, there was a graded relationship between peak troponin and 30-month outcomes (cardiovascular death, MI, or stroke).36
Within the past decade, conventional cTn assays have been gradually replaced with hs-cTn assays that can detect troponin at concentrations 10- to 100-fold lower.37,38 The benefits of hs-cTn include detection of myocardial injury at a shorter time interval from the onset of symptoms, and many studies have established the superiority of hs-cTn over conventional assays in evaluating patients presenting with chest pain.39–42 The 2021 AHA/ACC chest pain guidelines have a class I recommendation for hs-cTn being the preferred biomarker for patients presenting with acute chest pain due to its increased diagnostic accuracy.15 hs-cTn is a quantitative variable for cardiomyocyte injury, and therefore higher values increase the likelihood of the presence of MI.43 Elevations beyond fivefold the upper reference limit have a high positive predictive value (>90%) for ACS.44 Elevated measurements that occur without ACS are not necessarily “false positives”, but reflect acute myocardial injury in the setting of clinical entities beyond MI, including valvular heart disease, heart failure, hypertensive heart disease, chronic CAD, and myocarditis; because cTn is renally cleared, values may also be elevated in the setting of chronic kidney disease, especially end-stage renal disease.43,45,46
Although previous literature has established that hs-cTn allows the detection of ACS at lower thresholds, the implications on clinical outcomes are still debatable. In the High-STEACS trial, a cluster-randomized clinical trial across 10 hospitals in Scotland, implementing a cTn or hs-cTn assay in patients with suspected ACS demonstrated reclassification of 17% of patients with myocardial injury; however, one-third of patients had a diagnosis of type 1 MI, and there was no significant difference in the primary outcome of the incidence of subsequent MI or cardiovascular death at 1 year.47 hs-cTn led to a significant increase in detecting type 2 MI compared with a prior-generation assay, which points to the clinical challenge of distinguishing type 1 MI from the supply–demand mismatch that leads to type 2 MI.48 However, it is notable that patients with type 2 MI may have an even higher rate of cardiovascular mortality, and thus the recognition and treatment of such patients is essential.49 hs-cTn is highly reliable as a means to rule out acute myocardial injury and ACS, and low values portend a low risk (<1%) of 30-day MACE. For patients with acute chest pain that began at least 3 h before arrival at the ED, a single hs-cTn may be used to rule out myocardial injury (class IIa recommendation). CDPs based on hs-cTn that do not incorporate risk scores, such as the 2020 European Society of Cardiology (ESC) 0/1 h pathway, have been developed (Table 1) and are increasingly used in clinical practice.
Role of Anatomic or Functional Testing
Who Does Not Require Further Testing?
According to the 2021 AHA/ACC chest pain guidelines, it is a class I recommendation that patients presenting with acute chest pain who have a <1% probability of MACE within the next 30 days can be designated as low risk. These patients do not require admission for further cardiac testing.15 The guidelines have a class IIa recommendation that it is reasonable to discharge these patients home without further urgent workup. Nevertheless, further outpatient workup can be considered on an individual basis in these patients with low short-term risk. When a patient is identified as low risk, shared decision making is important to ensure clear communication. This is essential so providers can understand patients’ values and preferences, and so patients can understand their risk and why no further testing is recommended.15,50,51
Who Requires Further Anatomic or Functional Testing?
Intermediate-risk patients are a challenging group because they do not have a straightforward disposition from the ED, but rather require further evaluation in a dedicated observation unit or inpatient setting. The 2021 AHA/ACC chest pain guidelines differentiate intermediate-risk patients based on prior history of CAD, classifying patients as intermediate risk with known CAD or intermediate risk without known CAD.15 An echocardiogram is a class I recommendation for intermediate-risk patients because it can help evaluate ventricular and valvular function and assess new wall motion abnormalities.15 For intermediate-risk patients who do not have known CAD, the guidelines provide a class I recommendation for both anatomic testing, using coronary CT angiography (CCTA), and functional testing, using a stress echocardiogram, stress PET, stress single photon emission CT (SPECT), or stress cardiac MRI (CMR).15 Ultimately, the choice of test will be based on clinical expertise, availability, and patient characteristics that may favor specific testing approaches.
If the patient has had recent cardiac testing, the prior test results should guide the need for further workup. If a prior functional test had demonstrated moderate–severe ischemia and no known obstructive CAD established by prior anatomic testing, then proceeding to invasive coronary angiography (ICA) is recommended (class I). If the prior test was negative (within the past 1 year for functional testing and within the past 2 years for CCTA), further testing is not indicated and the patient can be discharged with outpatient follow-up. However, in that setting, it is important to ensure that the prior imaging was of sufficient quality with adequate levels of exercise or pharmacologic stress achieved based on the modality. In the case of an inconclusive or mildly abnormal functional test within the past year, CCTA is recommended (class IIa).15
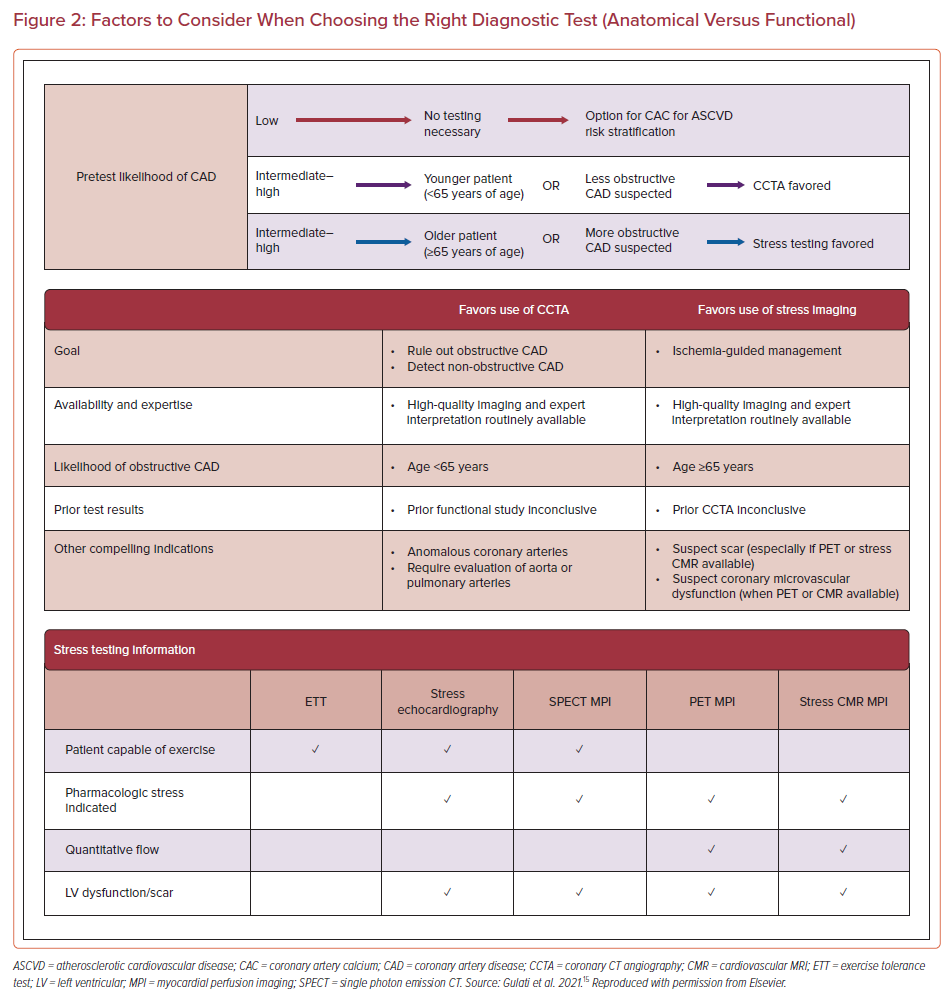
When selecting between anatomic and functional testing, it is essential to consider availability, expertise, and likelihood of obstructive CAD, as well as the overall clinical context of the patient (Figure 2). The 2021 AHA/ACC chest pain guidelines favor CCTA over stress testing in intermediate-risk patients who are younger and have a lower pretest probability of obstructive CAD, whereas in patients with a higher pre-test likelihood of CAD, such as older (>65 years) patients, functional testing is favored.15 Studies, such as ROMICAT, established the high negative predictive value of CCTA, in which 50% of patients with acute chest pain and a low to intermediate likelihood of ACS did not have any CAD by CCTA.52 Studies have demonstrated value in using CCTA to evaluate patients at low to intermediate risk presenting with possible ACS to guide the safe discharge of patients with non-obstructive CAD (<50% stenosis) or a completely negative (i.e. no plaque or stenosis) CCTA test.53–55 CCTA may identify up to 30% of patients with ACS presenting with positive troponin without obstructive CAD, and thus avoid the need for invasive angiography.56,57 However, in patients who are experiencing a definite MI (or who have known CAD), CCTA should be avoided because direct referral to invasive angiography would be preferable.58 In select cases, other imaging modalities, such as CMR, may be helpful in identifying the underlying cause for MI with non-obstructive CAD.59,60 In fact, the 2021 AHA/ACC chest pain guidelines provide a class I recommendation for CMR to distinguish myopericarditis from other causes, including MI and non-obstructive coronary arteries (MINOCA).15
In terms of choosing between various functional modalities, there may be specific indications that lead to preferences among the available options (Figure 2). For example, PET may be preferable because it has a higher accuracy and lower radiation dose than SPECT. PET can also be used to calculate myocardial blood flow reserve, which may be used to detect microvascular disease, evaluate the consequence of diffuse atherosclerosis, and improve the diagnostic and prognostic information provided by standard techniques used for myocardial perfusion imaging (MPI). However, this test is less available.61
The next step in the algorithm proposed by the 2021 AHA/ACC chest pain guidelines is dependent on the results of the test that was initially chosen.15 If the initial diagnostic test was inconclusive, then proceeding with another modality may be helpful if additional testing is required; for example, CCTA may be helpful after an inconclusive stress testing and vice versa. Another option when there are anatomical lesions of uncertain hemodynamic significance after CCTA is to proceed to fractional flow reserve (FFR)-CT, if available (class IIa recommendation).15 Specifically, patients may benefit from FFR-CT measurement if CCTA demonstrates 40–90% coronary artery stenosis in a proximal or middle coronary segment (class IIa recommendation). If stress testing shows moderate–severe ischemia, CCTA shows obstructive high-risk CAD, or FFR-CT shows moderate–severe ischemia, then further evaluation by ICA is warranted.
The discussion above pertains specifically to those intermediate-risk patients who do not have known CAD. For intermediate-risk patients with known CAD, the next branch point in the proposed algorithm is the presence of obstructive or non-obstructive CAD. Patients with non-obstructive CAD, defined as <50% stenosis, may undergo CCTA (class IIa recommendation) and can be discharged safely if CCTA shows no changes; however, if CCTA reveals obstructive CAD, further testing with functional stress testing or FFR-CT (if available) may be useful.
For patients with known obstructive CAD, if they have high-risk CAD (left main or proximal left anterior descending or multivessel CAD) or a history of coronary revascularization and present with frequent daily-to-weekly symptoms, they should proceed to ICA (class I recommendation). Otherwise, the guidelines have a class IIa recommendation for stress imaging in intermediate-risk patients with known obstructive CAD.15 If functional testing reveals moderate to severe ischemia, then ICA is warranted. As with low-risk patients, shared decision making for intermediate-risk patients was given a class Ib-R recommendation in the 2021 ACC/AHA chest pain guidelines, especially as it relates to the need for admission, observation, discharge, or further evaluation in an outpatient setting.15
Who Proceeds to Catheterization?
Patients presenting with chest pain and classified as high risk for ACS need urgent ICA because a delay in intervention can cause irreversible myocardial loss (class I recommendation).15 High-risk patients include those classified as high risk by CDP score, persistent chest pain, hemodynamic instability, elevated cardiac troponin confirming myocardial injury, new ischemic changes on ECG, new left ventricular systolic dysfunction (ejection fraction <40%), or newly diagnosed moderate–severe ischemia on functional testing (class I recommendation).15 ICA provides a comprehensive assessment of the extent and severity of obstructive CAD.
The GRACE risk model can also be used to identify high-risk patients who would benefit from early invasive strategy.62–64 In ACS patients with GRACE risk scores >140, the benefit of an early invasive treatment strategy has been shown in trials such as TIMACS and VERDICT.65,66 The data from these trials is reflected in the 2021 ACC/AHA/Society for Cardiovascular Angiography and Interventions guidelines for coronary artery revascularization and the 2020 European Society of Cardiology NSTE ACS guidelines, both of which recommend early invasive angiography in patients with high-risk NSTE ACS.44,67
For high-risk patients with acute chest pain and positive cTn or hs-cTn in whom obstructive CAD has been excluded by CCTA or ICA, echocardiography or CMR helps establish alternative diagnoses.68–72 In addition to high-risk patients, certain groups of intermediate-risk patients who present with chest pain, as described in earlier sections, also have a class I recommendation for ICA. This includes intermediate-risk patients with a prior history of high-risk CAD (left main or proximal left anterior descending or multivessel CAD) or a history of coronary revascularization who present with frequent daily-to-weekly symptoms, or patients with positive stress, CCTA, or FFR-CT tests.15 In addition, another group of patients with a class I recommendation for directly proceeding to invasive evaluation consists of those who have evidence of moderate or severe ischemia on previous stress testing and have not been revascularized previously; these patients have significant flow-limited CAD, so additional non-invasive stress testing will not be helpful in guiding further management.15
Specialized Care in Women and Diverse Patient Populations
Although advancements in diagnostic testing represent important innovations in the field of cardiovascular medicine, the clinical history remains of utmost importance in the diagnosis of ACS. The classic symptoms associated with ACS include retrosternal chest discomfort that gradually builds in intensity with characteristic radiation to the left arm, neck, or jaw and with associated symptoms, such as dyspnea and diaphoresis.73,74 However, it is important to consider that other associated symptoms may be more frequent among specific patient populations, including those with diabetes, women, and the elderly. The recent guidelines focus on the uniqueness of chest pain in women and have a class I recommendation that women are at risk for underdiagnosis, highlighting the importance of obtaining a history that emphasizes accompanying symptoms that are more common in women with ACS.15 Although studies demonstrate that men and women with ACS are equally likely to present with chest pain, the PROMISE trial, the YOUNG MI Registry, and the VIRGO trial demonstrated that women have a greater prevalence of associated symptoms such as palpitations, epigastric symptoms, and back pain.73,75,76
Furthermore, in order to improve the care of patients with ACS, it is imperative to address the disparities that exist in the care of diverse patient populations. The guidelines specifically recommend cultural competency training to achieve better outcomes in diverse patient populations and highlight the importance of addressing language barriers with the use of formal medical translation services for patients whose primary language is not English.15
Gaps in Knowledge
Risk stratification of acute chest pain is important to decrease mortality and morbidity; however, there are several challenges that remain. Although CDPs are helpful in separating patients into low-, intermediate-, and high-risk groups, their utility still needs to be validated in randomized trials to determine which CDPs lead to improved outcomes. In addition, as hs-cTn becomes the standard of care throughout institutions in the US, its use has raised questions regarding the significance of minimal elevations. Furthermore, although the chest pain guidelines provide specific recommendations regarding next steps based on a patient’s risk classification, there are less data available on how to choose between anatomic versus functional imaging modalities among high-risk patients; randomized trials comparing CCTA and different forms of stress testing are needed to further refine and optimize test selection.