Percutaneous intervention in cardiology typically relies on devices designed to be deployed in specific anatomies, for example, stents sized and deployed in coronary arteries, transcatheter heart valves implanted in calcified and failing valves, and occluding devices designed for the left atrial appendage or septal defects. In contrast, cardiothoracic surgeons rely on a toolkit to enable a variety of procedures that predominantly involve cutting and suturing. An important tool in the surgeon’s kit is the use of electrosurgery to cut and coagulate tissue. In this review article, we describe the novel percutaneous use of electrosurgery to enable transcatheter interventions.
Thermal cautery has been used for millennia, with the application of hot stones and iron pokers to treat maladies from battle wounds to boils. Electrocautery uses an electrical current to heat the instrument used to deliver thermal cautery to the treatment area, typically a superficial lesion. In contrast, electrosurgery transfers alternating current through the target tissue, which heats through energy generated from resistance, and is used for cutting or coagulation. In transcatheter electrosurgery, alternating current is directed through guidewires insulated from the surrounding blood pool.
The first reported use of transcatheter electrosurgery was in the treatment of pulmonary atresia, using an electrified guidewire to perforate through the atretic pulmonary valve.1 It has since been use to traverse occluded vessels, between cardiovascular chambers, particularly for interatrial septal puncture and transcaval large bore access, and for valve repair.2–12 A detailed review of these applications has recently been published.13
This review focuses on a novel application of transcatheter electrosurgery, that of tissue laceration. Transcatheter electrosurgery can be focused and controlled to cut heart valve tissue to enable transcatheter heart valve replacement.
The Cutting Edge of Transcatheter Electrosurgery
‘Cutting’ tissue requires sufficient charge concentration within the cells so they reach an internal temperature of 100°C, when intracellular water boils and the cells vaporize.
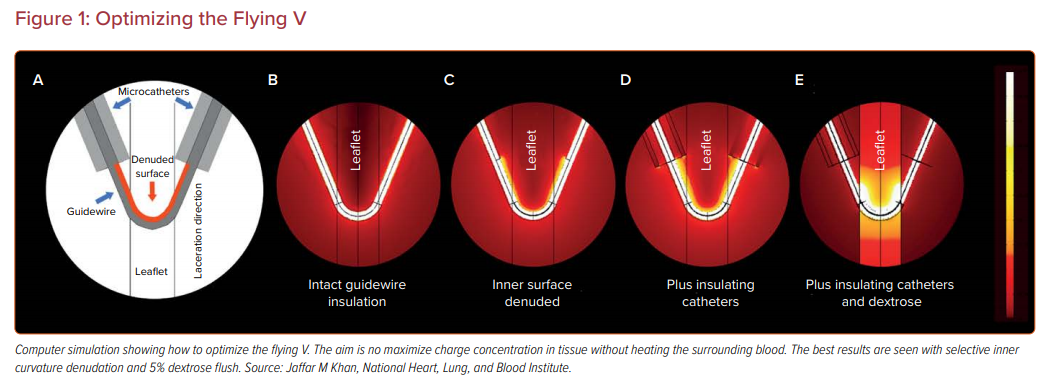
To achieve adequate charge concentration, a guidewire is modified to create a ‘flying V’ configuration, based on benchtop experiments and computer simulation, with insulating microcatheters and 5% dextrose flush (Figure 1).13 The midshaft of the guidewire is selectively denuded and kinked with a scalpel so that the current is preferentially conducted through the inner elbow.
The flying V is directed by catheters under imaging guidance to cut the target tissue. Often, but not always, the target leaflet is traversed at the base first, by electrifying the tip of the guidewire, and the flying V is saddled across the base and pulled towards the leaflet tip.
To date, there are three major applications of this technique to treat heart valve disease. The first is laceration of the anterior mitral leaflet to prevent outflow tract obstruction (LAMPOON). The second is bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA). The third is electrosurgical laceration and stabilization of a mitral edge-to-edge repair device (ELASTA-Clip).
Advances in LAMPOON
Transcatheter mitral valve replacement (TMVR) is a potential option for patients not suitable for surgery or transcatheter mitral valve repair.14 Transcatheter aortic valve replacement (TAVR) valves may be implanted in the mitral position inside a previous bioprosthetic mitral valve (MViV), off label inside a bioprosthetic ring (MViR) or in the native mitral annulus when there is severe mitral annular calcification (ViMAC). One dedicated mitral valve (Tendyne, Abbott) is licensed for use in Europe, and multiple transcatheter mitral valves are under investigational use in the US.
Left ventricular outflow tract (LVOT) obstruction is a common and fatal complication of TMVR. LVOT obstruction is commonest in ViMAC (40%), followed by ViR (5%) and ViV (2%), with a 30-day mortality of up to 62%.15,16 LVOT obstruction is a frequent reason for screen failure in TMVR trials.
During surgical mitral valve replacement, the anterior mitral leaflet is often resected down the midline with preservation of the chords to prevent LVOT obstruction. LAMPOON is a transcatheter mimic of the surgical standard. The technique was studied in an investigator-initiated prospective multicenter single-arm Food and Drug Administration (FDA) investigational device exemption clinical trial.17 LAMPOON traversal and laceration was technically successful in 100% of patients attempted, including in heavily calcified anatomies. LVOT obstruction was evident in only 3% on exit from the catheter laboratory despite the high risk in all. There were no strokes, and 30-day survival was 93%.
The LAMPOON technique has undergone a few iterations for ease of use in different anatomical situations and these are described below.
Retrograde LAMPOON
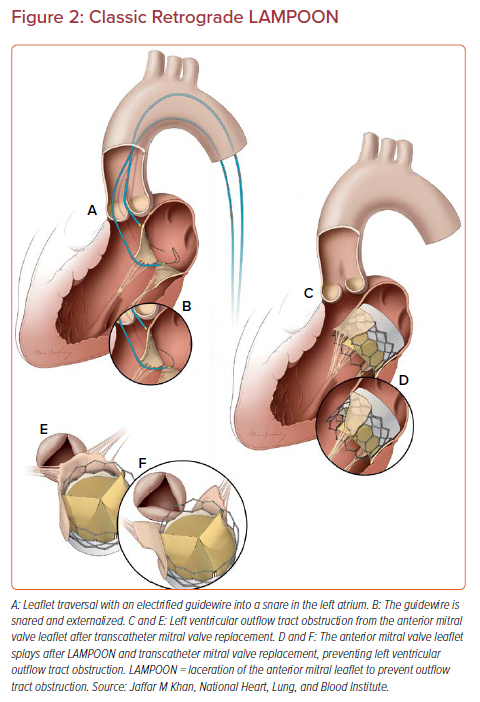
This is the original technique described and studied in the clinical trial.17–19 Two catheters introduced through femoral artery sheaths are positioned either side of the anterior mitral valve leaflet, with the traversal catheter in the LVOT and the snare catheter in the left atrium (Figure 2). An Astato XS 20 guidewire (Asahi Intecc) is sheathed in an insulating microcatheter (Piggyback Wire Converter, Teleflex). The back end of the guidewire is stripped and clipped to an electrosurgery pencil and generator.
Catheter position is confirmed on transesophageal echocardiography (TEE) and the guidewire is electrified for 1 second at 30–50 W in ‘pure cut’ mode while advanced through the center and base of the anterior mitral valve leaflet. The guidewire is snared in the left atrium. The microcatheter is withdrawn and the flying V is created at the midshaft of the guidewire. The tip is snare retrieved and the flying V advanced into the body and positioned to straddle the anterior mitral leaflet. Both guiding catheters are locked on to the guidewire by means of rotating hemostatic valves and torque devices, with only a few millimeters of guidewire exposed beyond the guide catheter tips. The guidewire is connected to the electrosurgery pencil and 70 W pure-cut power is delivered as the flying V is gently retracted, lacerating the leaflet down the centerline.
The advantage of this technique is that it reproducibly creates a central laceration in line with the LVOT, as this is the direction of pull on the catheters. It is also the technique that has been most extensively studied. The disadvantages are technical complexity in positioning the guide catheters for traversal and laceration, and that a mechanical aortic valve is a relative contraindication.
Antegrade LAMPOON
To treat patients with mechanical aortic valves, alternative routes for LAMPOON have been investigated.20 Apical LAMPOON has been performed in a handful of patients with mixed results (unpublished data). Transseptal LAMPOON has been performed with success, and was simpler in some respects than retrograde LAMPOON. Two deflectable sheaths are positioned in the left atrium through the same atrial septostomy. A balloon-wedge catheter is advanced through one deflectable sheath and floated through the main mitral orifice and into the LVOT, where it is exchanged over a rail wire for a guide catheter and snare. The second deflectable sheath directs a guide catheter to the center and base of the anterior mitral valve leaflet, guided by TEE. An Astato XS 20 guidewire is used to traverse the anterior mitral leaflet from left atrium to LVOT, where it is snared. The flying V is formed and positioned across the anterior mitral valve leaflet. The deflectable sheath tips are positioned in the center of the mitral orifice to act as a pivot for centerline laceration and the guide catheters are pulled into the sheaths during transcatheter electrosurgical laceration.
The advantages of this technique are the additional control afforded by transseptal access, as well as the ability to perform this in patients with mechanical aortic valves. There is a risk of eccentric laceration, which is mitigated by ensuring a good pivot from the sheaths in the left atrium.
Tip-to-base LAMPOON
In patients where there is a ‘backstop’ to prevent laceration of the aortomitral curtain and aortic root, for example, in patients with bioprosthetic valves or complete mitral rings, the traversal step can be omitted and mitral valve leaflet laceration can be performed from tip to base.20,21 A transseptal puncture is performed and a chord-free trajectory through the major mitral valve orifice and out of the aorta is established. The flying V is positioned, insulated by two guiding catheters. The position of the transeptal limb is adjusted to center on the mitral valve leaflet on TEE. Both catheters are pulled till the flying V comes to a hard stop against the bioprosthetic sewing ring.
Rescue LAMPOON
A long anterior mitral valve leaflet may cause LVOT obstruction even in the setting of a capacious neoLVOT. In the setting of dynamic LVOT obstruction from systolic anterior motion of the native mitral valve leaflet tip after TMVR, rescue LAMPOON can treat LVOT obstruction. The native anterior mitral leaflet tip extending beyond the implanted transcatheter heart valve frame is lacerated using the tip-to-base LAMPOON approach, with the transcatheter heart valve frame acting as the backstop.22
LAMPOON with Dedicated Transcatheter Mitral Valves
LAMPOON has been performed with the Sapien 3 valve (Edwards Lifesciences) when there is risk of either fixed LVOT obstruction from a small neoLVOT or dynamic LVOT obstruction from a long and redundant anterior mitral valve leaflet. The open valve frame in the left ventricle allows blood flow when the anterior mitral valve leaflet is cut and parted. Most of the dedicated mitral valve has a covered cell design so anterior mitral valve leaflet modification will not prevent fixed LVOT obstruction. However, LAMPOON can prevent dynamic LVOT obstruction from a long anterior mitral valve leaflet and has been successfully performed in a patient prior to Tendyne valve implantation.23
LAMPOON with Septal Modification When the Skirt neoLVOT is Small
The Sapien 3 valve is designed with only the distal cells uncovered. In certain anatomies, the LVOT is small and the covered skirt of the Sapien 3 is enough to cause LVOT obstruction.24 In cases where the predicted ‘skirt neoLVOT’ is small, preparatory transcoronary alcohol septal ablation or radiofrequency ablation of the septum is performed before LAMPOON and TMVR.25,26
Advances in BASILICA
Coronary Artery Obstruction
Coronary artery obstruction is a rare but devastating complication of TAVR, occurring in 0.7% of all cases, and 2.3% of valve-in-valve TAVR procedures, with an in-hospital mortality of 40–50%, even with attempted bail-out.27,28
Coronary obstruction occurs when diseased leaflets are displaced against the ostia of the coronary arteries or against the sinotubular junction (STJ). Prediction of coronary artery obstruction is imperfect. Those at higher risk have coronary artery heights <12 mm, sinus width <30 mm and virtual transcatheter heart valve to coronary distance (VTC) <4 mm.27,28 In addition, a virtual transcatheter heart valve to STJ distance <2 mm likely confers a high risk of coronary obstruction by sinus sequestration.29
Snorkelled stents are frequently underexpanded, unable to overcome the competing mechanical force from the transcatheter heart valve, and may be prone to immediate or delayed stent thrombosis.30,31 BASILICA is a transcatheter electrosurgery technique to lacerate and splay the aortic leaflets to prevent coronary artery obstruction. BASILICA has been investigated in a prospective single-arm multicenter FDA early feasibility clinical trial sponsored by the NHLBI.29 Single- and double-leaflet BASILICA was successful in 93% of patients enrolled. There was no coronary artery obstruction despite the high predicted risk in all. There was one death and disabling stoke (3%). There were no late events between 30 days and 1 year attributable to the BASILICA procedure (Khan et al. Circ Cardiovasc Interv, in press).
BASILICA Technique and Results
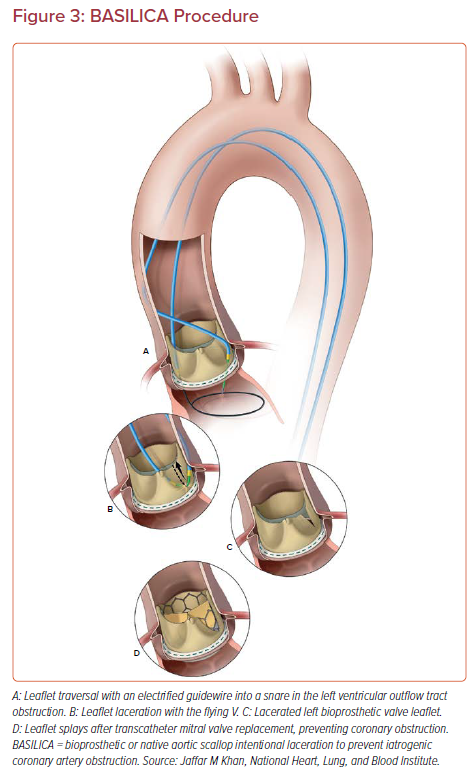
The BASILICA technique has been described in detail previously.32,33 Two guiding catheters are advanced from the femoral arteries and positioned either side of the target aortic leaflet (Figure 3). The catheter in the LVOT holds a snare, and the traversal guidewire and insulating microcatheter are advanced through the catheter in the aortic root. The guidewire is briefly electrified at 30–50 W while it is advanced through the center and base of the aortic leaflet into the LVOT. The guidewire is advanced further without electrification through the snare. The microcatheter is withdrawn and the flying V created. The snared tip is then retrieved, and the flying V is introduced into the body and positioned across the aortic leaflet. A 5% dextrose flush is injected through both guiding catheters and the guidewire is electrified at 70 W while traction is applied to both limbs till the leaflet is completely lacerated down the centerline.
If double-leaflet BASILICA is planned, both flying Vs are formed and positioned, then laceration is performed sequentially. TAVR is performed as usual, with extra care being taken to land the skirt of the TAVR valve below the level of the threatened coronary ostium.
TAV-in-TAV
Coronary artery obstruction may become a problem of epidemic proportion as younger patients are getting TAVR and are likely to require a second or third valve in their lifetime. In CT analyses of patients after TAVR, TAV-in-TAV greatly increases the risk for coronary artery obstruction.34 For Sapien 3 valves, the predicted risk for coronary obstruction with second TAVR was 13% in a CT analysis of the Low Risk TAVR trial.35 For Evolut valves (Medtronic), the predicted risk of coronary obstruction with a second TAVR was 23%.36
Clinical experience of BASILICA to prevent TAV-in-TAV coronary obstruction is limited. In preclinical modeling, leaflet splay in newer generation transcatheter heart valves appeared to be less effective, which potentially limits the applicability of the technique to selected cases.37
Pachyderm Catheters
Dedicated BASILICA guide catheters have been designed for left and right leaflet BASILICA. Pachyderm guiding catheters (Medtronic). help direct the traversing guidewire through the base of the left and right leaflets and into the LVOT. They have been shown to decrease procedure time compared with coronary guide catheters.38
ELASTA-Clip: Burning the Bridge
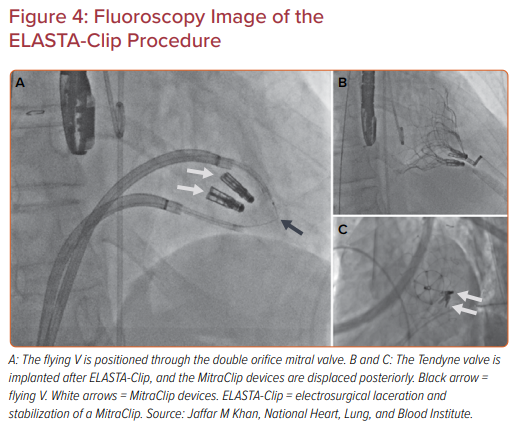
Edge-to-edge mitral valve repair with a clip device (MitraClip, Abbott) may ‘burn the bridge’ to downstream therapy, particularly TMVR, as a result of the double orifice created. Patients who develop mitral stenosis, with or without residual or recurrent mitral regurgitation after edge-to-edge repair, have few options. ELASTA-Clip is a simple concept, where the anterior leaflet attachment of the clip device is cut using transcatheter electrosurgery, effectively burning the bridge and creating a single orifice to enable TMVR (Figure 4).39,40
The procedure was performed in five patients on a compassionate basis who had failed MitraClip implantation and had no other options.40 Two guiding sheaths were positioned in the left atrium through the same atrial septostomy. Guide catheters were advanced through the medial and lateral orifices either side of the MitraClip devices. An Astato guidewire was advanced and snared between the two guide catheters and the flying V formed and positioned across the MitraClip.
Under fluoroscopy and TEE guidance, the flying V was moved to the anterior attachment of the MitraClip devices. The flying V was then tensioned and electrified at 70 W under 5% dextrose flush to liberate the MitraClip devices from the anterior mitral valve leaflet. The clips remained attached to the posterior leaflet. Transapical Tendyne valve implantation was then performed as usual. The patients remained hemodynamically stable between laceration and TMVR, with elective use of an intra-aortic balloon pump upfront in all cases.
A key learning point from this experience, alongside a demonstration of feasibility, was that detaching MitraClip devices acutely increases mitral valve diameter so Tendyne valve oversizing achieves optimal results.
Future Directions
Transcatheter electrosurgery increases the versatility of percutaneous intervention. The ability to create controlled cuts in cardiac tissue using completely percutaneous techniques moves us closer to truly minimally invasive surgery. By understanding the principles and applications, tailored therapies are possible for unique anatomies. Dedicated devices will make these procedures more accessible and reproducible, and are currently in development.
All the techniques described are advanced procedures and should be undertaken with appropriate proctoring at experienced centers.
Conclusion
Transcatheter electrosurgery is at the cutting edge of structural heart intervention. It is a versatile tool and has been applied to enable TAVR when there is risk of coronary artery obstruction, TMVR when there is a risk of LVOT obstruction, and TMVR in the setting of previous edge-to-edge repair.