ICDs were developed more than 40 years ago and approved for use by the Food and Drug Administration (FDA) in 1985. They have remained a cornerstone of treatment in the prevention of sudden cardiac death ever since. Early ICDs were larger in size than those used now, and were placed subcutaneously in the abdomen. With the development of the transvenous-ICD (TV-ICD) system, the smaller pulse generator size and transvenous leads have allowed for placement of the device subcutaneously within the pre-pectoral space.
Despite the progression in ICD technology, there are still important risks and complications associated with traditional TV-ICDs. Some of the more serious complications include systemic infection (often lead related), pneumothorax, lead perforation or malfunction. To minimize such risks, the subcutaneous ICD (S-ICD) has emerged as a potential alternative therapy in the prevention of sudden cardiac death, with increasing use in the US. Studies of the first-generation S-ICDs showed high inappropriate shock rates but low complication rates, with high success for terminating ventricular arrhythmias.1
S-ICDs have been studied in multicenter clinical trials for more than a decade. Data have shown high complication-free rates and high shock efficacy for S-ICDs. Despite these apparent advantages, S-ICDs have limitations and potential risks too, including inappropriate shock delivery, lack of pacing and CRT therapy, and shorter device longevity. For instance, a recent report from Boston Scientific identified that its EMBLEM S-ICD Subcutaneous Electrode (Model 3501) body fractures just distal to the proximal sense ring, with an occurrence rate of 0.2% at 41 months.2
This review will summarize the rationale for S-ICDs in the appropriate patient population, in the context of the most relevant literature.
Subcutaneous ICD Leads
A significant limitation of conventional ICDs is the implantation of transvenous leads, which lend themselves to a variety of potential complications.3 Implant complications include pneumothorax, hemothorax, and cardiac tamponade.4 More chronic complications of TV-ICDs include infection and lead malfunction caused by insulation breaks or fracture, often exposing patients to inappropriate ICD shocks. Such shocks can also occur as a result of myopotentials and T wave oversensing.3–11 Lead failure often requires revision and/or extraction, and there are significant complications associated with these procedures.4
In contrast to TV-ICDs, vascular access is preserved with S-ICD implants, because the leads are placed subcutaneously. This eliminates many of the potential risks previously outlined. Furthermore, fluoroscopy is not required during the procedure because insertion of the S-ICD system is guided by anatomical landmarks only.4,5 The S-ICD pulse generator is placed in a lower left lateral thorax position, between the anterior and mid-axillary lines near the apex of the left ventricle.3 Attached to the generator is a single lead with a shock coil, which is tunneled from the lateral pocket medially to the xiphoid process and positioned 1–2 cm left of the sternum, with the distal tip close to the manubriosternal junction.3,12
The structure of S-ICD leads also allows for enhanced durability in comparison with transvenous leads. S-ICD leads do not require a stylet for placement and have no central lumen, which allows for higher tensile strength.3 Longer follow-up is still needed to adequately to assess longevity of S-ICD leads.3 Currently, the longest follow-up reported is 5.8 years, according to the European Registry Trial, during which no lead malfunctions or failures were noted.3,13 However, as highlighted above, a unique fracture point of the lead has been noted, leading to an FDA recall.14
Infection
Device infection is a problem associated with TV-ICDs and S-ICDs. However, it is important note that S-ICD infections are not associated with endocarditis and bacteremia.15,16 The EFFORTLESS registry and IDE trials reported no S-ICD infections associated with bacteremia.10,17
In a study by Bardy et al., the authors designed and tested an entirely S-ICD system evaluating four S-ICD configurations in 78 patients. They reported pocket infections in two of 55 patients enrolled in the European clinical trial.4 Higher infection rates have been reported in other studies, such as the IDE trial. Among the 330 patients in the IDE trial, 18 (5.5%) had suspected or confirmed infections reported by principal investigators. Four of these infections required explant, and the remaining 14 were considered superficial or incisional infections (4.4%).10
Reported infection rates have varied considerably between retrospective analyses. There were higher rates noted in studies by Jarman et al. and Olde Nordkamp et al., reporting infection in 11 of 111 (9.9%) and in seven of 118 implants (5.9%), respectively.3,6,8 However, other studies, such as by Abenkari et al., report lower infection rates of 3.2%.5 Part of the wide variability in reported events is the lack of consensus on the classification of infection events. Nevertheless, American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines recommend S-ICD over TV-ICDs for those at higher risk of infection.18 S-ICD remains a class I recommendation for patients at high risk of infection or without adequate venous access, without an indication for pacing or antitachycardia pacing (ATP).18,19
Site Complications
Achieving venous access can often prolong procedure times during TV-ICD placement. Venous access is not used for S-ICD implantation but site complications such as hematoma and device erosion can still occur, although they are rare.3,4 In a study by Köbe et al., one out of 69 patients (1.4%) reported hematomas.9 Most other studies, including the combined IDE and EFFORTLESS S-ICD registries, had much lower rates at 0.4% and 0.2%, respectively.3,17,20
With regard to the risk of device erosion, large pulse generator size and implant location have been problematic. First-generation S-ICDs, in particular, were significantly larger than TV-ICDs. The highest rates of device erosion have been reported by Jaman et al., noting an erosion rate of 18.8%.3,11 Most of the other early studies reported much lower rates at 1.4% and 0.8%.17,20 Others have reported no device erosions in their study cohort.4–7,9,10 The erosion rate was <0.2% with the small second- and third-generation devices.11 Moreover, erosion rates may be reduced further with newer implant techniques placing the device partially intermuscularly.21 In addition to this approach, the less invasive two-incision technique, as described by Knops et al., is a safe and efficacious alternative for S-ICD implantation as it avoids a third, superior parasternal incision.22
Efficacy
Trials have reported high success rates for tachyarrhythmia detection and shock efficacy for S-ICDs. Bardy et al. demonstrated successful detection and treatment of all 12 episodes of spontaneous, sustained ventricular tachyarrhythmias.4 Induced VF was detected in 100% of 137 induced episodes. VF was converted in 58 out of 59 patients (98%) with delivery of 65 J shocks in two consecutive tests.4 Other trials also demonstrate accurate ventricular tachycardia (VT)/VF detection and high rates of conversion, with first shock efficacy of 95.2% for monomorphic VT and 86.7% for polymorphic VT.23,24
S-ICD shocks for spontaneous arrhythmias are non-programmable at 80J, but reverse polarity with successive shocks if additional shocks are required for successful defibrillation.25 Failure of conversion with the first shock is predicted by patient height and BMI.26 As noted by Bardy et al., defibrillation threshold energy of S-ICDs is roughly three times higher than TV-ICDs (11.1 J versus 36.6 J).4 Despite this, the 80 J shock delivery of S-ICDs allows for a greater defibrillation threshold (DFT) safety margin.
In their retrospective analysis, Do et al. also noted that higher BMI and body surface area, and increased posterior and septal wall thickness, are associated with elevated S-ICD DFTs.27 It has been shown that patients with hypertrophic cardiomyopathy (HCM) have higher DFTs, and a higher percentage of HCM patients have DFTs >20 J with transvenous devices, which increases as left ventricular (LV) wall thickness increases, although other studies have shown high success rates in patients with HCM.28,29 In a large analysis from multicenter trials, S-ICD patients with HCM have comparable outcomes to non-HCM patients. Specifically, in a cohort of 99 HCM patients versus 773 non-HCM patients, successful defibrillation at >80J was achieved in 98.9% of HCM and 98.5% of non-HCM patients. Overall shock conversion efficacy was 100% in HCM versus 98% in non-HCM patients (p=not significant).30 These data suggest that S-ICD can be safe and effective for patients with HCM. This was further supported in the study by Francia et al., which showed that contemporary ICDs are safe and effective in HCM patients independent from LV hypertrophy.31
In addition to body habitus, S-ICD implant strategy and position are important determinants of S-ICD efficacy. Posterior generator and coil positioning have been associated with lower DFTs. High impedance is also associated with inadequate coil depth and lower rate of defibrillator success.32,33 Brouwer et al. compared various implantation techniques and found that the two-incision technique was a feasible alternative to the three-implantation technique and associated with shorter procedure times.34
In the IDE study, chronic conversion testing (>150 days post implant) was performed as a secondary endpoint as a surrogate to examine post-implant effectiveness. A 96% success rate with 65 J shock and 100% with 80 J shock were reported.10 In addition, S-ICDs demonstrated a 92.1% first shock success rate with 100.0% overall conversion rate among 119 spontaneous ventricular arrhythmia episodes in 21 patients, which is similar to rates noted in transvenous studies.10,35
Inappropriate Shocks
Inappropriate shocks as a result of oversensing or errors in arrhythmia discrimination had been one of the main drawbacks of S-ICDs with early generations of the device and programming. Jarman et al. reported inappropriate shocks in 17 of 22 patients. However, not all studies describe rates that high. Rates of 2.5% and 4.0% have been reported by Burke et al. and Köbe et al., respectively.9,20 In general, rates have ranged from 5% to 25% in early S-ICD trials.4–11 In contrast, TV-ICDs rates of inappropriate shock delivery with contemporary programming are <5%.3,36
High shock rates in early S-ICD trials were often a result of T wave oversensing, lead migration, or supraventricular tachycardias (SVTs) at rates in the shock zone where discrimination algorithms are inactive. In a comparison of TV-ICD and S-ICD sensing algorithms for discrimination of arrhythmias induced at implant, the START trial demonstrated equivalent S-ICD detection of ventricular arrhythmias and improved discrimination of supraventricular arrhythmias.25 Two strategies to mitigate inappropriate S-ICD shock occurrence have been the software update with enhanced SVT discrimination and device reprogramming. Earlier generations of S-ICDs were often programmed with a shock only zone at a rate of 180 BPM. However, the use of a conditional zone, where discriminators are active, in addition to a higher rate shock zone, improved discrimination. The importance of the conditional zone for discrimination was apparent in analyses of prospective trials and is now the standard programming used.37
Developments in programming of both S-ICDs and TV-ICDs have led to reduction in inappropriate shock rates. Studies, such as ADVANCE III and MADIT-RIT, have shown that prolonging detection reduces shock rates in TV-ICDs.36,38 Although detection duration is not programmable in S-ICDs, the charge time of the device and the discrimination algorithms inherently prolong detection. Moreover, improved filtering of electrograms, known as SMART Pass, was developed. This technology has been shown to reduce first inappropriate shock rates by 50%, and risk for all inappropriate shocks by 68%.39
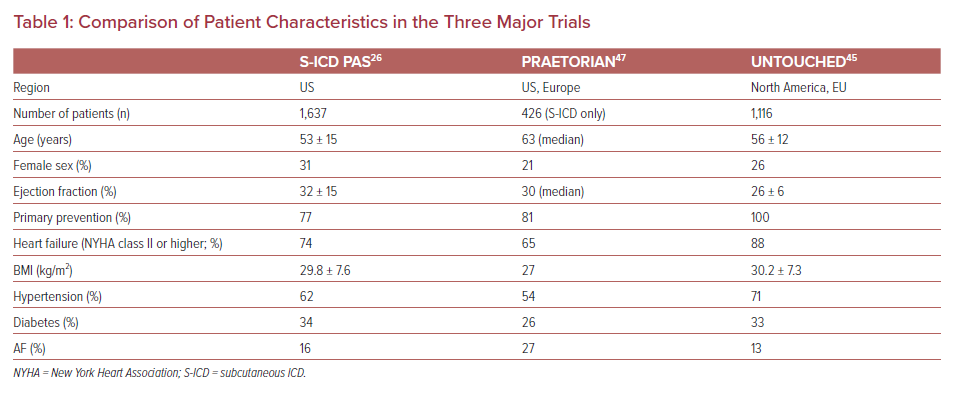
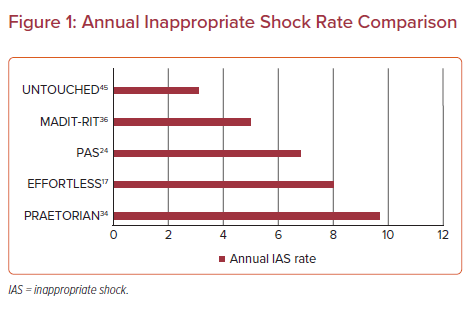
Earlier studies, such as EFFORTLESS and IDE, enrolled younger, healthier patients and demonstrated higher inappropriate shock rates. One of the first studies to enroll a sicker patient cohort with more comorbidities was the Subcutaneous ICD PAS study. This was an FDA-mandated registry of US S-ICD patients. This study demonstrated a complication free rate of 92.0% and an appropriate shock rate of 5.3%.24 Along with the subsequent trials, UNTOUCHED and PRAETORIAN, the PAS study illustrates low complication rates and high success rates of ICD therapy in patients without a pacing indication.24 Furthermore, the inappropriate shock rate has progressively declined from the PRAETORIAN to PAS to the UNTOUCHED trial, which reported the lowest rate of any prior multicenter S-ICD trials. This has been largely because of the development of improved programming and the SMART Pass filter.24,39 Table 1 shows the comparison of baseline characteristics among three of the major clinical trials. Figure 1 shows the comparison of annual inappropriate shock rates between some of the major trials.
Lack of Pacing Ability
One of the major limitations of S-ICDs is the lack of pacemaker capability. Unfortunately, this limits its use in the advanced heart failure population, as QRS prolongation, which may benefit from CRT, is an important component of therapy for this cohort. According to data from the European Regulatory Trial cohort, one of 55 patients (1.8%) developed an indication for bradycardia pacing over a 5.8-year follow-up period, thus requiring S-ICD explant.40 In this same cohort, two of 55 patients (3.6%) developed symptomatic heart failure and underwent explant of their S-ICD in exchange for a transvenous CRT.40 Pacing in transvenous devices is also used for painless termination of VT. Such ATP is not available in the S-ICD, but explant of the device for this indication is uncommon, probably because of exclusion of patients with known monomorphic VT at rates likely to be pace terminated. This confirms that proper patient selection can minimize the need for subsequent device upgrade for pacing. In contrast, retrospective studies have suggested that many patients with TV-ICDs would be appropriate for S-ICDs.41 Of note, even when bradycardia cardiac pacing requirements develop, there have been small case series which have successfully implemented concurrent use of S-ICDs and transvenous pacemakers, as opposed to S-ICD explant.3 However, this requires careful assessment of crosstalk between devices.42,43 To date, there have not been any large-scale studies to evaluate the safety and efficacy of this approach.
Preoperative Screening
In contrast to TV-ICDs the S-ICD requires preoperative EKG screening, which is largely designed to assess the ratio of R wave and T wave amplitudes to reduce the incidence of T wave oversensing. This was initially performed manually and could be time consuming. An automated programmer based screening tool has now been developed, which has similar performance to manual screening.44 However, there are little prospective data on the use of this tool in multicenter studies. For instance, only 28% of subjects in the UNTOUCHED study underwent automated screening.45
UNTOUCHED Trial
The UNTOUCHED trial was designed as a multinational, prospective trial to investigate limitations of S-ICDs in a higher-risk population of patients. Study subjects across 110 sites (>1,110 patients) were enrolled. Those with LV ejection fractions (LVEF) ≤35%, as a result of either ischemic or non-ischemic etiologies, were included in the study. Patients with pacing or cardiac resynchronization therapy indications, history of sustained ventricular arrhythmias or New York Heart Association classification. Patients underwent standard pre-implant screening and the programming of therapy zones was mandated. Specifically, the conditional zone was programmed at 200 BPM and the shock zone at 250 BPM. The primary endpoint for the study was the inappropriate shock free rate at 540 days (18 months), compared with a performance goal of 91.6% derived from the MADIT-RIT cohort.45,46 The performance goal was developed based on results of ICD patients in the active arms of the MADIT-RIT TV-ICD study excluding CRT devices.45,46
At 18 months, the inappropriate shock-free rate was 95.9%, meeting the performance goal of 91.6%. In multivariate models, patients with history of AF (paroxysmal, persistent or permanent), non-ischemic etiology, and those with a lower EF had higher risk of inappropriate shocks. According to regression analysis, predictors of inappropriate shock included history of AF and two-incision implant technique (as opposed to three-incision implant). Moreover, the complication-free rate was 95.8% at 30 days, compared with the performance goal of 93.8%. Despite a cohort with higher LV dysfunction and heart failure, the UNTOUCHED trial outcomes demonstrated the lowest ever inappropriate shock rate compared with prior S-ICD and ICD trials.45,46
PRAETORIAN Trial
The PRAETORIAN trial was a randomized, controlled, multicenter, prospective two-arm trial comparing safety and efficacy of S-ICDs with TV-ICDs. The main objective was to assess noninferiority of the S-ICD compared with TV-ICD in terms of inappropriate shocks and major complications among patients with a class I or IIa indication for an ICD and without a pacing indication.47 The study spanned approximately 7 years and included 876 patients. The study population had a median LVEF of 30%. Over a 50-month follow-up period, the incidence of inappropriate shock was higher in the S-ICD group, which was primarily a result of cardiac oversensing. However, this difference was not statistically significant. The rate of complications was higher in the TV-ICD group but again this did not achieve statistical significance so noninferiority was established for the two arms of the study. Lead complications were higher with TV-ICDs. Notably, appropriate ICD shocks were higher in the S-ICD group, as the system is not capable of delivering ATP. In the TV-ICD group, the rate of ATP was higher, and successfully terminated 55% of all treated ventricular arrhythmias.47 With regard to the safety endpoint, lead complications were significantly more common in the TV-ICD arm.
Conclusion
Studies such as PRAETORIAN and UNTOUCHED demonstrate favorable outcomes of S-ICD in a traditional, higher-risk patient population, comparable to that of typical TV-ICD cohorts. The S-ICD was developed to reduce potential complications often associated with TV-ICDs and this was observed in these trials.48–50 Moreover, inappropriate shock rates are now much lower with later generation devices.
Despite the promising results of S-ICD trials, S-ICDs have their own limitations and potential complications. One limitation of S-ICD is the lack of pacing functionality, thus limiting its use in patients with bradycardia, CRT or ATPneeds. Trials of an extravascular ICD with pacing capabilities or leadless pacemakers to communicate with S-ICDs to address these limitations are ongoing. However, battery life is still shorter than for single chamber TV-ICDs.
Large prospective trials of the S-ICD show that this device remains safe and effective among more traditional ICD patients for both primary and secondary prevention. The studies support the use of all ICD indicated patients in the absence of pacing indications. In this regard, clinical studies have recently reported a new device capable of pacing the heart from a substernal location, and the use of a leadless pacemaker combined with an S-ICD.51,52