In the US, the annual direct cost of heart failure (HF) is estimated at over $30 billion, largely driven by the >1 million hospitalizations in which HF is the primary discharge diagnosis.1–3 The subset of patients with HF with reduced ejection fraction (HFrEF) comprises about half of all patients with HF.4 Guideline-directed medical therapy (GDMT) is proven to reduce mortality and morbidity for patients with HFrEF. GDMT includes the following drug therapies: renin-angiotensin-aldosterone system inhibitors (RAAS-I), with or without a neprilysin inhibitor, β-blockers, and mineralocorticoid-receptor-antagonists (MRA).5
Recently, sodium-glucose cotransporter-2 inhibitors (SGLT2i) demonstrated efficacy as an important fourth pillar of GDMT.6 Together, this combination can add over six additional years of lifespan for HFrEF patients compared to the traditional approach of RAAS-I and β-blockers alone.7 However, studies highlight that many eligible HFrEF patients are not receiving one or more of the recommended medications, in the absence of contraindications or intolerance.8 Even among patients who are treated, less than half receive optimal doses of GDMT. Additionally, time to initiation and optimization of dosing may be exceedingly slow in the outpatient setting.
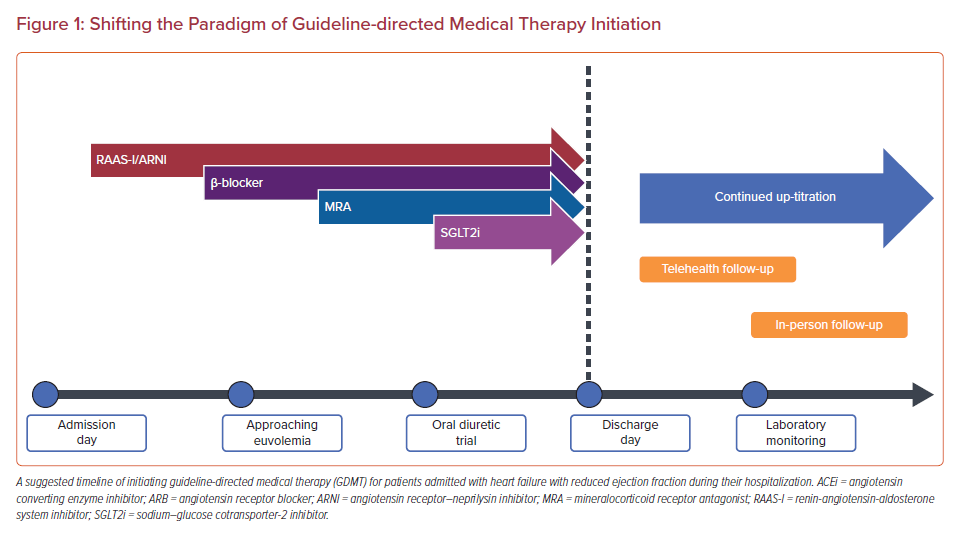
Analysis of the CHAMP registry showed that less than 1% of patients receive target doses of RAAS-I/angiotensin receptor–neprilysin inhibitor (ARNI), β-blockers, and MRA simultaneously over a 12-month period.8,9 Guideline recommendations for device therapy, such as ICD and cardiac resynchronization therapy (CRT), recommend consideration only after 3 months of GDMT optimization. Yet, target doses of GDMT are rarely achieved prior to device therapy implantation.5,10 Barriers to optimization include lack of access and coordination of care, gaps in provider knowledge, and patient-related factors such as renal function, perceived intolerance to medications, inadequate insurance coverage, out-of-pocket expenses, and adherence.11,12 Hospitalization provides an opportunity to initiate and titrate medical therapy with close monitoring in patients with new-onset or acute on chronic HFrEF.13 Medications provided at discharge are more likely to be adhered to, continued, and further titrated in the outpatient setting.14 An acute heart failure (AHF) hospitalization is an important opportunity to initiate and titrate all four pillars of GDMT (Figure 1), laying a complete foundation for further outpatient optimization. We present a practical guide for the initiation and titration of evidence-based chronic HFrEF therapies during hospitalization.
Renin-Angiotensin-Aldosterone-Inhibitor/Angiotensin Receptor–Neprilysin Inhibitor
According to expert consensus, optimization of GDMT in an AHF hospitalization should occur once a positive clinical trajectory has been obtained.15 Although initially studied in the outpatient setting, angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) have been shown in multiple observational studies to be safely and effectively initiated during hospitalization for AHF, with associated reduction in rehospitalization and mortality.16,17
ARNI is preferred to RAAS-I alone given substantial mortality benefit, similar tolerability, and long-term cost-effectiveness compared to ACEi or ARB.18–20 The PARADIGM-HF trial showed a 16% all-cause death reduction and 21% hazard reduction for HF first hospitalization in the ARNI group compared to the ACEi group.21 Of the patients entering the ARNI ‘run-in’ phase of the trial, less than 5% dropped out as a result of hypotension, renal dysfunction, and hyperkalemia – the three most common reasons for pre-trial drop-out.22 Patients who were then randomized to ARNI actually had lower rates of discontinuation compared to those randomized to ACEi.21 Importantly, symptomatic hypotension is the most common adverse effect of ARNI initiation and occurs about 15% of the time; it is less commonly seen with ACEi or ARB.21,23 A detailed review by Sauer et al. provides further practical guidance on ARNI use.24
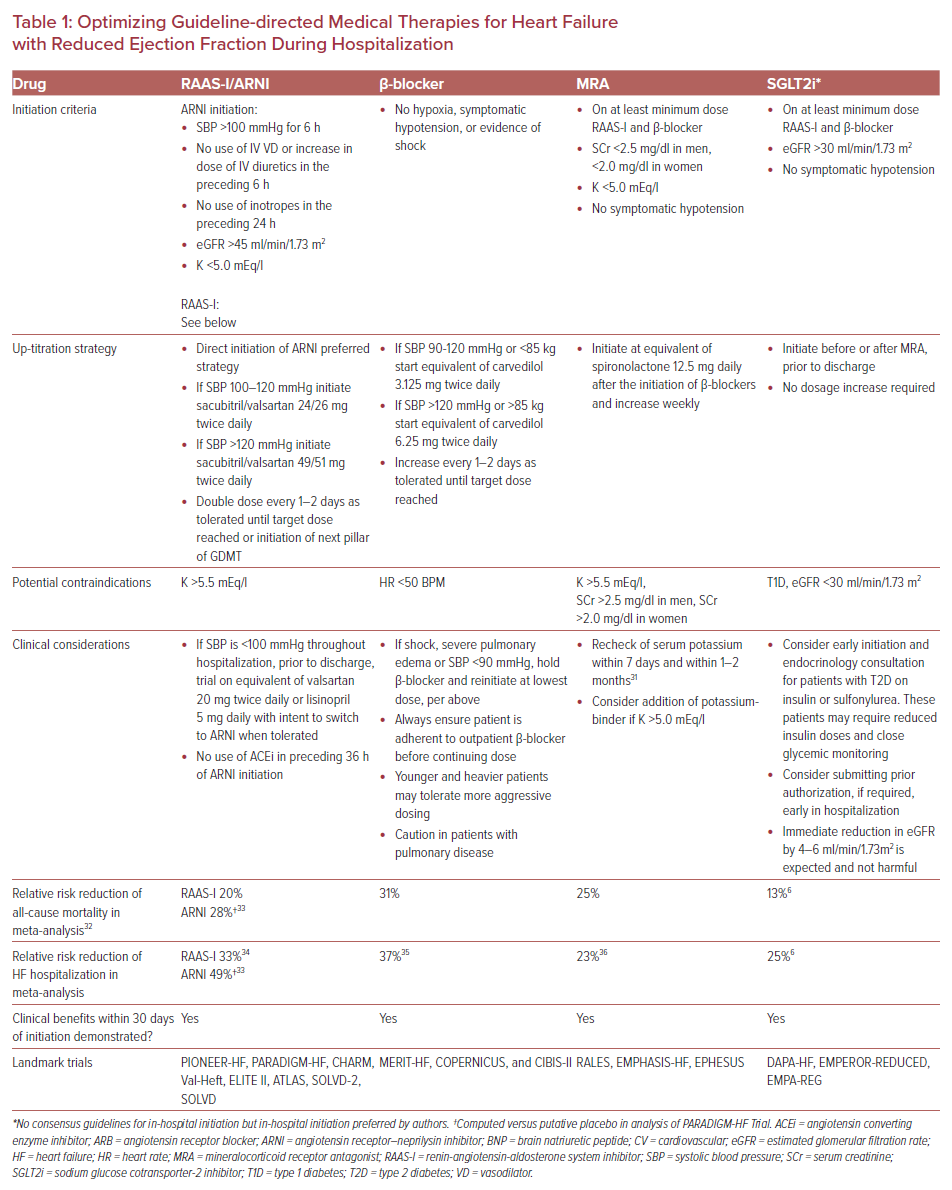
The PIONEER-HF trial suggested benefits of ARNI initiation during hospitalization greater than that achieved with ACEi.23 Analysis of the trial showed a 31% lower hazard for cardiovascular (CV) death or rehospitalization events for patients that were initiated on an ARNI during hospitalization versus those initiated on the ACEi, enalapril, in the first 8 weeks.25 PIONEER-HF showed that an ARNI can safely be initiated in patients with a systolic blood pressure (SBP) >100 mmHg for 6 hours, no use of vasodilators or increase in dose of intravenous diuretics in the preceding 6 hours, and no use of inotropes in the preceding 24 hours.23
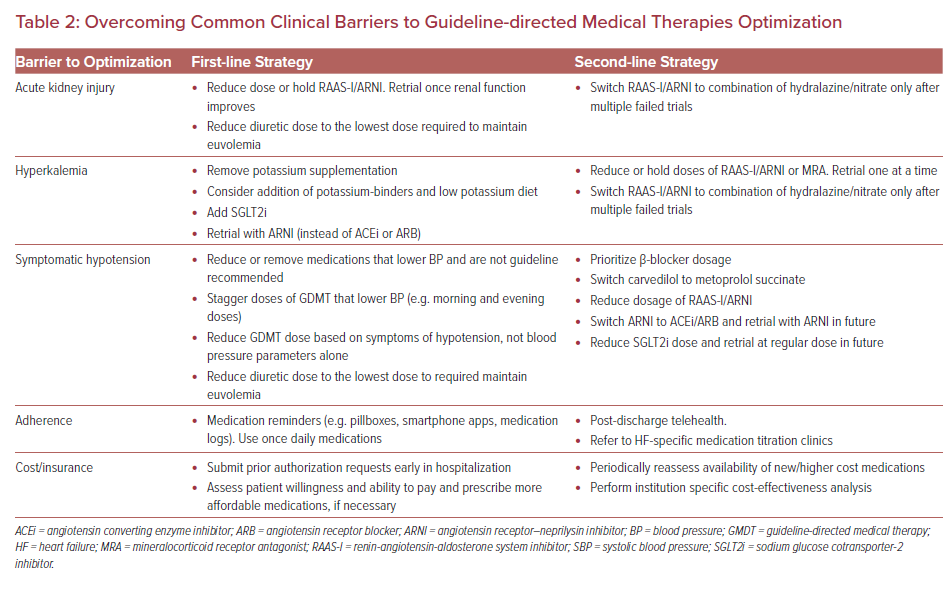
Contrary to popular belief, RAAS-I/ARNI initiation is well tolerated in cases of mild renal dysfunction, for example, the interquartile range (IQR) of baseline estimated glomerular filtration rate (eGFR) in PIONEER-HF was 47.1–71.5 ml/min/1.73m2.23,26,27 Acute kidney injury (AKI) after starting RAAS-I occurs in ~13% of HF patients but the long-term overall benefit of RAAS inhibition tends to outweigh any reduction in renal function, which is often transient.28 Experts agree that RAAS-I/ARNI should be continued if serum creatinine (SCr) increases <30% after initiation.29 For changes in SCr >30%, RAAS-I/ARNI doses can be reduced or temporarily held to assess for improvement in renal function. Once renal function begins to improve, RAAS-I/ARNI should be re-trialed to assess maximally tolerated dose.
RAAS-I show modest dose responsiveness. High-dose RAAS-I (equivalent to lisinopril 20–40 mg daily) results in a 6% decrease in all-cause mortality compared to low-dose RAAS-I (equivalent to lisinopril 5–10 mg daily).30 This effect is similar for ARNI with maintained advantage over ACEi or ARB even at lower doses.31 Clinicians should aim to initiate a RAAS-I, preferably an ARNI, as soon as clinical stability has been obtained. Table 1 shows our proposed strategy of RAAS-I/ARNI initiation.
β-blockers
In three landmark trials, β-blockers demonstrated a reduction in all-cause mortality by 34–35%, even among patients with severe HF or recent decompensation.32–34 In-hospital initiation of β-blocker therapy for HFrEF has been shown to be safe, well tolerated, and associated with reduced mortality within the first 30 days of hospital discharge.35 This was observed even in patients with signs and symptoms of congestion. β-blockers show the clearest dose response relationship among GDMT.36 Initiating or up-titrating a β-blocker during hospitalization is an effective strategy to increase the likelihood of long-term target dose attainment.37 Martinez-Sellez et al. showed the safety of early initiation of carvedilol in AHF patients at a median 3 days after admission, achieving a mean discharge dose of 23 mg/day among those who tolerated in-hospital up-titration.38 No study has definitively shown the superiority of one evidence-based β-blocker versus another.39 Carvedilol may have a more potent blood pressure lowering effect versus metoprolol succinate in those with more elevated blood pressure.40 Although, for patients with lower blood pressure at baseline, β-blockers are hemodynamically well tolerated.41 In COPERNICUS, patients with a systolic blood pressure (SBP) of 85–95 mmHg at baseline had an increase in SBP with carvedilol greater than placebo.
β-blockers should not routinely be discontinued on admission for AHF. Multiple studies have shown that discontinuation of β-blockers in AHF is associated with worsening short- and long-term mortality, although hypotension/shock or severe pulmonary edema may represent indications for temporary discontinuation.42 Otherwise, as long as the patient confirms adherence prior to admission, β-blockers can be safely continued during hospitalization.
β-blockers should be initiated or up-titrated so long as the patient is hemodynamically stable without marked volume overload, typically after the initiation of a RAAS-I/ARNI.43 Younger patients with higher BMI and without chronic obstructive pulmonary disease tend to tolerate higher doses and may be appropriate for a higher starting dose or more aggressive up-titration.44 Up-titration in patients may occur as tolerated toward target dose prior to discharge (Table 1).
Mineralocorticoid Receptor Antagonist
Only one-third of patients indicated for MRA receive them.8 Use of these agents has reduced CV death and HF hospitalization, with benefits observed within 30 days of initiation.45 Addition of MRA to RAAS-I and β-blockers has been shown to be safe during hospitalization.46 Further, long-term adherence is substantially improved with in-hospital initiation compared to delaying therapy to outpatient at clinician discretion.47 Hospitalization represents an opportunity to safely initiate an MRA under close monitoring, with provider fear of hyperkalemia and renal function often cited as barriers to initiation.48 Potassium binding drugs such as patiromer and sodium zirconium cyclosilicate have shown to be effective long-term options to maintain normokalemia in HF patients.49 While these agents do not confer mortality benefit on their own, they are beneficial in patients who would have otherwise been unable to tolerate MRA therapy. Additionally, combined usage of MRA with SGLT2i or ARNI (versus ACEi) lowers the risk of hyperkalemia.50,51 At the dosages commonly used in trials, MRAs do not have any blood pressure lowering effect and can be safely initiated on borderline hypotensive patients.52 There is no clear dose response relationship with MRA, although the target dose in major trials was the equivalent of 25–50 mg/day of spironolactone.53 We suggest initiating an MRA after RAAS-I/ARNI and β-blocker initiation, but prior to discharge (Table 1).
Sodium-Glucose Cotransporter-2 Inhibitor
The DAPA-HF and EMPEROR-Reduced trials showed the significant benefits of SGLT2i in the treatment of patients with HFrEF, including reductions in all-cause mortality risk by 13% and recurrent CV hospitalizations by 25% in HFrEF patients with or without type 2 diabetes (T2DM).6 These clinical benefits can be seen within 12 days of initiation.54 Additionally, the DAPA-CKD trial showed a 44% decrease in the composite of a sustained decline in eGFR of at least 50%, end-stage kidney disease, or death from renal causes.55 Similar results were found in the CREDENCE trial.56 With an estimated 54% of HFrEF patients having concomitant CKD and declining renal function as one of the strongest predictors of mortality in HFrEF, SGLT2i hold substantial clinical promise as a vital fourth pillar of GDMT.57
The novelty of SGLT2i, along with their potential impact on glycemic control for patients with T2DM contribute to relatively low rates of prescription for HFrEF compared to more established pillars of GDMT.58,59 An eGFR <30 ml/min/1.73 m2 is one of a few exclusions to starting the medication at this time, but more than 80% of HFrEF patients are eligible for treatment with SGLT2i.7 SGLT2i can be safely initiated during hospitalization and do not need to be up-titrated from its initial dosage. Sotagliflozin, a dual SGLT-1 and -2 inhibitor, displayed benefits within 1 month of initiation when started during or shortly after hospitalization.60–62
SGLT2i do exhibit a modest BP lowering effect in HF patients (~1–4mmHg).63,64 Additionally, attention should be paid to glycemic management when initiating SGLT-2i in patients on sulfonylureas or insulin due to risk of hypoglycemia and euglycemic diabetic ketoacidosis.64 Volume status of patients on diuretics should be reevaluated due to the risk of over-diuresis. Honigberg et al. previously described in detail the practical considerations for the initiation of SGLT2i. Given the rapid onset of clinical benefit, we favor in-hospital initiation of SGLT2i.65 A summary of the important points for in-hospital initiation are presented in Table 1.
Hydralazine/Nitrate
Although the hydralazine/nitrate combination was the first treatment to show mortality benefit in HFrEF, its usage varies widely.66,67 Current guidelines recommend a hydralazine/nitrate combination for New York Heart Association (NYHA) class III/IV self-described African-American patients who are optimized on RAAS-I and β-blockers.5 Because of modest mortality benefits and relatively larger blood pressure effects, the hydralazine/nitrate combination does not need to be prioritized for in-hospital initiation and is not one of the critical four pillars of therapy.67,68 In patients who cannot take RAAS-I/ARNI due to renal dysfunction, hyperkalemia, or drug intolerance, the hydralazine/nitrate combination can be considered as alternate therapy, regardless of race.5 However, because of the demonstrated superiority of RAAS-I/ARNI, a repeat trial with a RAAS-I/ARNI should be attempted when deemed safe.68
Loop Diuretic
Diuretic optimization is an important aspect of chronic HF treatment for symptom management and reducing the likelihood of hospitalization for worsening HF.69 Three loop diuretics are commonly used for the treatment of HF: furosemide, bumetanide and torsemide.70 No trials have definitively shown superiority of one versus the other; however, furosemide is most commonly used in clinical practice and generally has the lowest acquisition cost.71,72 In a meta-analysis, torsemide has been shown to reduce hospitalizations, lower cardiac mortality, and improve functional status compared to furosemide.73 Torsemide also has the longest half-life and offers the advantage of consistent diuretic effect with once daily dosing.71 Both torsemide and bumetanide have superior bioavailability to furosemide. Guidelines recommend trialing an oral diuretic prior to discharge to verify dose effectiveness.5 In a study comparing patients observed on at least 24 hours of an oral diuretic regimen prior to discharge versus those not, 30-, 60-, and 90-day HF readmissions were reduced significantly.74 Fluid restriction should be liberalized for an oral diuretic trial to better match the diuretic dose with the patient’s unrestricted fluid intake.75 The lowest effective dose of loop diuretic should be used to maintain euvolemia, allowing for maximal up-titration of GDMT.71
Transition of Care
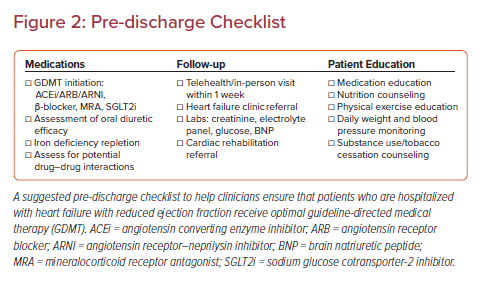
Although the average length of stay for an AHF admission is 5 days, an early focus on GDMT during the admission can increase the likelihood of initiation of all four agents, especially with increasing system pressures to reduce time in hospital.76,77 Following discharge, maintaining momentum and ensuring safety of in-hospital initiation and titration of GDMT is dependent on successful transition of care to the outpatient setting. A systematic review identified eight common components of successful programs: “telephone follow-up, education, self-management, weight monitoring, sodium restriction or dietary advice, exercise recommendations, medication review, and social and psychological support.”78 Supervised exercise training programs (cardiac rehabilitation) for at least 8 weeks following discharge reduce mortality and rehospitalization.79 Iron deficiency should be treated prior to discharge, with one dose of intravenous iron prior to discharge and additional doses as an outpatient shown to improve symptoms and reduce risk of rehospitalization.43,80 Additionally, laboratory values, particularly renal function markers, potassium, and glucose, should be monitored within 1–2 weeks of discharge to evaluate for adverse reactions to GDMT initiation.5 Brain natriuretic peptide (BNP) or N-terminal pro-hormone BNP (NT-proBNP) levels obtained at 1–2 months after GDMT initiation can be useful predictors of rehospitalization and response to therapy.18,81 An assessment of left ventricular systolic function by echocardiography should be performed ~3 months after optimal GDMT has been reached to assess response to therapy and indication for ICD implantation.43 Further, the coronavirus disease 2019 pandemic has highlighted the opportunities to integrate telehealth to continue the aggressive up-titration of GDMT with the use of specialized nurses and pharmacists.82 A suggested pre-discharge checklist is presented in Figure 2 and strategies to overcome common clinical barriers are presented in Table 2.
Conclusion
Given the clinical benefits seen within 30 days of initiation of RAAS-I/ARNI, β-blockers, MRA, and SGLT2i, combined initiation of these four foundational therapies is preferable to the outdated paradigm of pursuing maximally tolerated β-blockers and RAAS-I prior to the addition of secondary agents. Additionally, due to the severe lack of optimization of GDMT in the outpatient setting, in-hospital initiation and titration of the four agents should be attempted in all patients admitted for AHF. The ability to closely monitor in the hospital setting allows for more aggressive up-titration and early recognition of adverse effects. Our strategy prioritizes the implementation of all four pillars of GDMT to meet the evolving standard of optimal treatment of HFrEF.