Coronary CT angiography (CCTA) is a robust non-invasive method for direct visualization of the coronary arteries and atherosclerotic plaque burden. Recent advancements in cardiac CT and its clinical application have enabled the production of high-quality images with low radiation exposure. CCTA has a high sensitivity and high negative predictive value for the detection of coronary artery disease (CAD). The main limitations of CCTA are the low specificity and positive predictive value for determining the severity and hemodynamic significance of coronary stenosis.1,2 The functional significance of coronary stenosis dictates the prognosis and the need for coronary revascularization in patients with stable CAD.3
Functional ischemia was found in less than half of the patients referred for invasive coronary angiography (ICA) based on the angiographic severity of stenosis.4,5 Such limitations of CCTA have raised the concern that it could lead to unnecessary ICA or revascularization procedures for patients who do not have ischemia. This led to the introduction of fractional flow reserve (FFR) derived from conventional CCTA (FFRCT) to determine the physiologic significance of CAD, in order to reduce the false-positive rate and incidence of negative referrals to ICA. FFRCT, a non-invasive method, is the gold standard diagnostic method for guiding decision-making to identify stable CAD patients who would benefit from revascularization. In this article we review the role of FFRCT according to supporting evidence and describe the challenges in its widespread application for determining hemodynamically significant stenosis, based on the most significant research articles.
Real-world Experience With FFR
ICA has routinely been used to detect coronary artery plaques to determine the need for revascularization, independent of quantitative coronary angiographic modalities. However, the vast majority of patients referred for ICA have either been discharged with no evidence of CAD (54–62%) or have undergone revascularization of lesions that are not hemodynamically significant or that are not the true cause of the symptoms.6
Various methods have been developed and are now available in the catheterization laboratory to determine the functional significance of coronary lesions. The most acceptable measure of the hemodynamic pressure of coronary stenoses is FFR, which aids the interventionist to identify specific vessels and lesions that require appropriate revascularization. FFR is measured routinely in the catheterization laboratory using a pressure wire with an IC or IV vasodilator to produce maximal hyperemia. FFR represents the fraction of the normal maximal myocardial flow across the coronary stenotic lesion, and an FFR of 0.75 represents a stenosis causing a 25% drop in pressure across the lesion. Deferral of percutaneous coronary intervention (PCI) has been shown to be safe in patients with >50% visual stenosis on ICA but an invasive FFR ≥0.75.7 Invasive FFR during PCI reduces the composite outcome of death, non-fatal MI, and revascularization in patients with stable multivessel CAD.8 However, FFR is not routinely measured in clinical practice due to the invasive nature of the FFR procedure, the added time, the use of radiation and contrast, the cost of adenosine needed during FFR measurement, the high cost of the pressure-sensing wires, and the limited reimbursement.8–11 For instance, invasive FFR was performed in only 6.1% of patients according to data from more than 60,000 ICA cases in the National Cardiovascular Data Registry. Of these 6.1%, FFR procedures were more likely to be performed in a university hospital setting than in private and community hospitals (p<0.0001).12
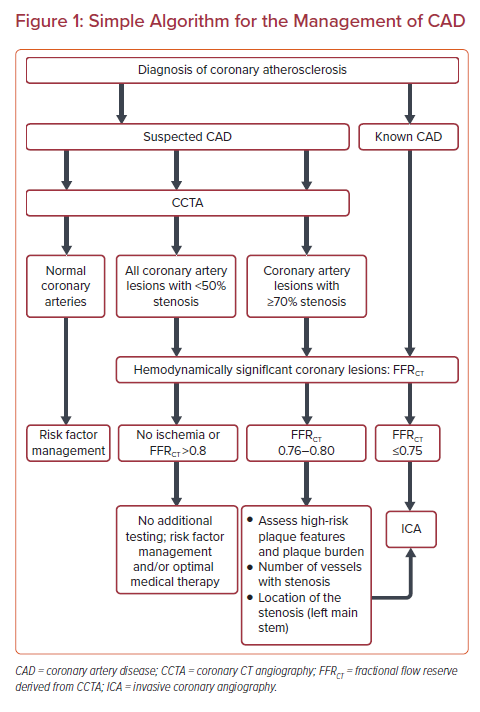
In studies on the use of CCTA in individuals with suspected CAD and the indications for ICA, the prevalence of significant CAD on CCTA was reported in only 23% (CAT-CAD study) and 53% (CONSERVE study) of the study populations.4,13 Given this superior performance of CCTA, prior knowledge of the functional significance of coronary artery lesions before angiography may reduce the need for invasive procedures and the healthcare cost. Non-invasive FFRCT fills that gap and provides the scores of hemodynamic pressure and flow across the entire coronary tree. Invasive FFR, however, assesses only the pressure gradient in the targeted vessel chosen during ICA at the discretion of the interventionist. There is great value in measuring FFRCT after stress testing. In the National Cardiovascular Data Registry, after abnormal functional test, only 47% were found to have obstructive CAD.14 Use of FFRCT can cut down on false-positive stress tests by at least 80% (PLATFORM STUDY).15,16 Kim et al. reported that CT-derived computer modeling is feasible and helpful to predict functional outcome after coronary stenting.17 FFRCT had a 96% diagnostic accuracy in predicting or ruling out myocardial ischemia after stenting, with a mean difference of 0.02 ± 0.05 between FFR after stenting and FFRCT after virtual stenting. Here, we present a simple algorithm for the management of CAD (Figure 1).
In the case of high-risk patients with chronic kidney disease, it is prudent to proceed directly to the catheterization laboratory to avoid double doses of contrast, which may cause contrast-induced nephropathy.
Overview of Non-invasive FFRCT
FFRCT was first proposed by Charles Taylor and colleagues, and it involves the application of computational fluid dynamics to the available anatomical data from CCTA to produce a 3D model of coronary blood flow and pressures.18,19 A minimum of 64-slice CCTA is required to produce the data for FFRCT analysis, and the currently available method is marketed by HeartFlow. FFRCT enables calculation of rest and hyperemic pressure fields in coronary arteries without the use of additional medication (i.e. adenosine), additional imaging or radiation exposure, or changes to CCTA protocols.20
The steps involved in the computation of FFRCT are based on the Navier–Stokes equations, the physical laws that govern fluid dynamics, which have been previously published.3,21–23 The physiologic model is derived using the patient’s anatomical model and is based on three scientific principles. The first principle is that resting total coronary blood flow is proportional to myocardial mass from volumetric CCTA. The second principle is that the total coronary resistance is calculated from the inverse relationship between microcirculatory resistance at rest and vessel diameter. The third principle is that the vasodilatory response of the coronary microcirculation to adenosine is able to be predicted.
The precise interpretation of CCTA and FFRCT depends on image quality, especially in patients with high heart rate, arrhythmias and other artifacts. Recent advances in CCTA and its clinical application, however, means that high image quality is now available, with temporal spatial resolution and software-based motion correction.
Diagnostic Accuracy of FFRCT
CCTA is reported to be only moderately predictive of abnormal invasive FFR, which has become the standard reference for the identification of clinically significant lesions of stenosis. The DEFER and FAME studies showed a low risk of adverse cardiovascular outcomes associated with the FFR-based revascularization procedure.5,24,25 FFR, the ratio of blood flow through a coronary artery with stenosis to that through a coronary artery without stenosis, is equal to 1.0 in a normal coronary artery, while FFR ≤ 0.80 identifies ischemia-causing coronary stenoses with >90% accuracy.25 FFRCT is a novel method that has been reported to reduce the need for ICA and the healthcare cost.26
Three major, prospective, multicenter studies have evaluated the diagnostic performance of FFRCT in patients with suspected or known CAD, using invasive FFR as the reference method. First, the DISCOVER-FLOW (Diagnosis of Ischemia-causing Stenoses Obtained via Non-invasive Fractional Flow Reserve) study enrolled 103 patients, 56% of whom had ≥1 vessel with FFR ≤ 0.80.18 Using receiver operating characteristics (ROC) analysis, a higher area under the curve (AUC) was noted for FFRCT relative to CCTA (0.90 versus 0.75; p=0.001). Second, the DeFACTO (Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography) study demonstrated only 54% specificity on a per-patient basis, which did not meet the pre-specified primary endpoint for diagnostic accuracy of >70% for the lower boundary of the 95% CI.27 The specificity in the per-vessel analysis was improved to 61% with high sensitivity (80%), but this was still low compared with DISCOVER-FLOW.27 The DISCOVER-FLOW study showed good correlation with invasive FFR, but the DEFACTO study failed to achieve a similar accuracy. The third study was the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps), which enrolled 254 patients who were clinically referred for ICA for suspected CAD.28 CCTA was performed before ICA. On a per-patient basis, the sensitivity and specificity to identify myocardial ischemia were 86% and 79% for FFRCT, versus 94% and 34% for coronary CTA, and 64% and 83% for ICA, respectively. In predicting functionally important CAD, FFRCT was found to have greater value than CCTA on a per-patient (AUC 0.9 versus 0.81; p=0.0008) and per-vessel level (AUC 0.93 versus 0.79; p=0.0001). FFRCT reclassified 68% of patients with false-positive CCTA, 67% of whom were found to have true-negative results.28 In a meta-analysis by Gonzalez et al., FFRCT was observed to have a significantly higher specificity compared with CCTA (72% versus 43%; p=0.004) on a per-patient basis, with a high positive predictive value (70%).29 No improvement in the sensitivity was reported, however, and the per-vessel analysis did not show a significant correlation for either sensitivity or specificity between CCTA and FFRCT. However, when Xu et al. compared the integrated results from DISCOVER-FLOW and DeFACTO with the data from the NXT trial (which used a refined version of FFRCT), a significant improvement was seen in specificity on a per-patient basis with the upgraded FFRCT technology (62.2% versus 78.7%, p<0.001).30
Multiple studies have confirmed the superior diagnostic value of CCTA with the addition of FFR in identifying functionally significant lesions.25–30 FFR analysis further improves the specificity and positive predictive value of CCTA, with high sensitivity and negative predictive value. The accurate interpretation of FFRCT completely relies on the quality of the CCTA images, especially in patients with high heart rate, arrhythmias and other artifacts. Even a large calcified lesion may pose a challenge for the 3D anatomical modeling required for FFRCT analysis. However, the discrepancies between FFRCT and invasive FFR due to CT image quality and use of sublingual nitroglycerin or β-blockers prior to CCTA, can be accounted for using CTA-derived computational algorithms. Also, the recent advancements in CT technology with regard to enhanced spatial and temporal resolution and iterative image reconstruction, mean that improved image quality can be achieved even in the case of calcified lesions or motion artifacts. A DISCOVER-FLOW substudy assessed the effect of CT image quality on the accuracy of FFRCT and noted the superior accuracy of FFRCT over CCTA even in the presence of coronary calcification, motion artifacts and low signal-to-noise ratio.31 Leipsic et al. reported an improved diagnostic performance of FFRCT with the use of β-blockers and nitroglycerin prior to CCTA, however, the diagnostic accuracy of FFRCT was significantly reduced with misalignment artifact (accuracy, 56.0% versus 71.0%, p=0.03; sensitivity, 43.0% versus 86.0%, p=0.001).32 Furthermore, Nørgaard et al. showed that the diagnostic efficiency of FFRCT was not reduced compared with CCTA in patients with high coronary artery calcium score ≥400.33
The FFRCT technique uses computational fluid dynamics principles and image-based modeling from CCTA images to non-invasively determine the coronary flow and pressures along the length of the entire coronary tree. The evidence-based studies of the diagnostic performance of FFRCT are summarized in Supplementary Material Table 1. According to the evidence-based studies, FFRCT diagnostic accuracy ranges from 73% to 81%, and FFRCT has a sensitivity of 86–93% and a specificity of 54–79%. In summary, compared with invasively measured FFR, non-invasive FFRCT has a high diagnostic accuracy for the detection of ischemia-causing stenosis in stable patients with suspected or known CAD.
Impact on Clinical Decision-making and Prognostic Value of FFRCT
In addition to the enhanced prognostic value relative to CCTA stenosis, FFRCT may have a direct impact on therapeutic decision-making, which further enhances the efficiency of ICA in patients with suspected CAD. The evidence-based studies are summarized in Supplementary Material Table 2. FFRCT may change the downstream management of patients by identifying individuals with stenosis and calcification.34,35 In the FFRCT RIPCORD study, 47% (n=94) of all the participants had significant obstructive CAD on CCTA and were later found to have no obstructive disease on ICA. Of these 94 patients, 57 (60.6%) had FFRCT >0.80 and 37 (39.4%) had FFRCT ≤0.80. This discrepancy between the physiological significance and visual assessment of stenosis severity resulted in a change in the allocated management plans for 72 (36%) of the patients.36 Similarly, PLATFORM (Prospective Longitudinal Trial of FFRCT: Outcome and Resource Impacts) found no obstructive CAD on ICA in 24 (12.4%) of the CTA/FFRCT arm or in 137 (73.3%) of the invasive arm participants (risk difference 60.8%; 95% CI [53.0–68.7]; p<0.0001).6 In the patients in the CTA/FFRCT arm who were scheduled for planned ICA, there were only two major adverse cardiac events (MACEs: all-cause mortality, MI, and unplanned hospitalization for chest pain leading to urgent revascularization) were reported, which was too low to determine the significance of the correlation. In the ADVANCE (Assessing Diagnostic Value of Non-invasive FFRCT in Coronary Care) registry, 72.3% of the study population undergoing invasive angiography with FFRCT ≤0.80 underwent revascularization.15,16 Non-obstructive coronary disease (no stenoses >50%) was significantly lower in ICA patients with FFRCT ≤0.80 compared with patients with FFRCT >0.80 (OR 0.19; 95% CI [0.15–0.25]; p<0.001).
FFRCT enables assessment of the hemodynamic forces that play a role in the pathogenesis of atherosclerotic plaque.37,38 In order to select the appropriate treatment, it is essential to identify the number of vessels that need intervention in patients with multivessel CAD and to identify the location of the cause of lesion-specific ischemia. The anatomic SYNTAX (Synergy between PCI with Taxus and Cardiac Surgery) score was proposed to determine the complexity of atherosclerotic lesions based on location, severity, bifurcation and calcification.39,40 The integration of anatomic, plaque and hemodynamic characteristics can aid in non-invasive risk prediction of acute coronary syndrome. The SYNTAX III study demonstrated that CCTA alone overestimated the anatomic SYNTAX scores compared with FFRCT, and that FFRCT had good diagnostic accuracy compared with invasive functional SYNTAX scores.7,8 Furthermore, the anatomic and functional information derived from FFRCT to calculate SYNTAX scores aids clinicians in deciding between optimal medical therapy and revascularization, and between PCI and coronary artery bypass graft surgery.9,40 There were no serious adverse cardiac events in patients who were deferred from ICA based on FFRCT.10 An appropriate revascularization strategy, involving planning and the selection of target lesion for revascularization, is possible with FFRCT, which can provide both anatomic and functional information prior to the invasive procedure.
FFRCT Versus Other Non-invasive Functional Tests
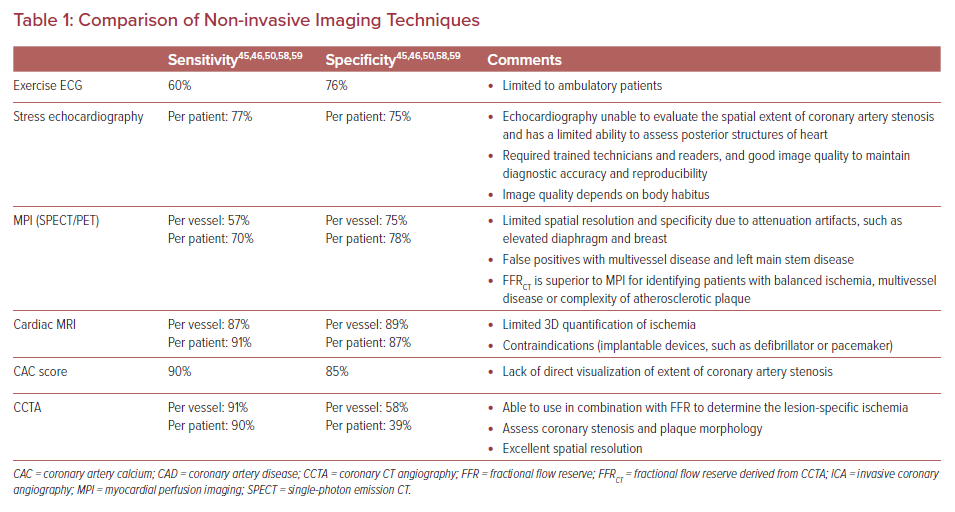
The use of FFRCT for the detection of lesion-specific ischemia was compared with that using other functional cardiac testing. The advantages and disadvantages of various non-invasive modalities used for the assessment of coronary ischemia have been previously published and are summarized in Table 1.41 In comparison with myocardial perfusion imaging, CCTA has the ability to detect, localize and exclude CAD accurately.42,43 CCTA, however, overestimates the severity of lesions and does not define their functional significance reliably. This gap in non-invasive testing could be resolved by integrating anatomic and physiologic data. Yang et al. demonstrated the increased diagnostic accuracy of CCTA when combined with FFRCT (AUC 0.856 versus 0.919; p=0.004), and showed that CT-derived FFR significantly correlated with invasive FFR (r=0.671, p<0.001).44
Pontone et al. evaluated the diagnostic accuracy of CT perfusion plus CCTA compared with FFRCT plus CCTA and reported no significant difference in sensitivity, specificity or AUC (p=0.4).45 However, the diagnostic performance for the detection of lesion-specific ischemia was improved when CCTA was combined with CT perfusion plus FFRCT compared with CCTA plus CT perfusion (AUC 0.919 versus 0.876; p=0.016).45 A significant correlation of per-vessel FFRCT to invasive FFR was reported (Pearson’s correlation coefficient 0.69; p<0.001). FFRCT also provided additional value in terms of specificity, positive predictive value and diagnostic accuracy to detect flow-limiting stenosis (patient-based AUC 0.90 versus 0.94; vessel-based AUC 0.89 versus 0.93; p<0.001) compared with CCTA alone. Li et al. have demonstrated the low specificity (63% versus 91%; p<0.001) and AUC (0.79 versus 0.96, p<0.001) of machine learning-based FFRCT compared with myocardial blood flow derived from CT perfusion for detecting hemodynamically significant CAD.46 The investigators reported that CT perfusion delivered higher radiation doses compared with FFRCT (3.6 ± 1.1 mSv versus 2.7 ± 0.8 mSv). Additionally, Nørgaard et al. investigated the association of moving from myocardial perfusion imaging to FFRCT testing with the downstream utilization of diagnostic and therapeutic ICA.47 In patients referred to ICA, the rate of non-obstructive CAD was reduced by 12.8% (95% CI [−22.2, −3.4]; p=0.008) and the rate of revascularization increased by 14.1% (95% CI [3.3–24.9]; p=0.01). Downstream ICA utilization (after adjusting for baseline risk factors) was also reduced by 4.2% (95% CI [−6.9, −1.6]; p=0.002) with an FFRCT strategy.
Sand et al. compared the per-patient diagnostic performance of FFRCT with that of single-photon emission CT (SPECT).48 FFRCT had a higher sensitivity than SPECT (91% versus 41%; p<0.001) and reclassified six patients with multivessel disease with false-negative SPECT. The specificity of FFRCT was low compared with SPECT (55% versus 86%; p<0.001), and the authors attributed this to the vasodilatory response due to the use of nitroglycerine tablets rather than spray in the CT image acquisition protocol. The post-hoc substudy of the PACIFIC (Prospective comparison of cardiac PET/CT, SPECT/CT Perfusion Imaging and CCTA with ICA) trial included 208 patients and compared the accuracy of FFRCT to PET and SPECT.49 On a per-vessel level, FFRCT had a high sensitivity compared with CCTA (90% versus 68%, p<0.001), SPECT (90% versus 42%, p<0.001) and PET (90% versus 81%, p=0.03).49 The AUC for identifying ischemia-causing lesions was significantly greater for FFRCT compared with CCTA (p<0.01) and SPECT (p<0.01) on both per-vessel and per-patient analysis. PET had the highest per-patient and per-vessel AUC, followed by FFRCT in the intention-to-diagnose analysis (0.86 versus 0.83; p=0.157; and 0.90 versus 0.79; p=0.005, respectively). However, FFRCT has the superior clinical diagnostic ability to provide anatomically and functionally significant information on coronary lesions in patients with multivessel disease.49
In a meta-analysis of 23 studies, Danad et al. compared the accuracy of various non-invasive imaging modalities (cardiac MRI, echocardiography, SPECT, CCTA) to that of FFRCT using invasive FFR as the reference standard for ischemia.50 Forest plots at the per-patient level showed a high sensitivity for FFRCT (90%; 95% CI [85–93]), CCTA (90%; 95% CI [86–93]) and cardiac MRI (90%; 95% CI [75–97]) with a low sensitivity for SPECT (70%; 95% CI [59–80]) and ICA (69%; 95% CI [65–75]). The highest specificity was noted for cardiac MRI (94%; 95% CI [79–99]) and the lowest for CCTA (39%; 95% CI [34–44]), with intermediate specificity for SPECT, echocardiography, FFRCT, and ICA. On a per-vessel basis, low diagnostic performance was observed for SPECT, stress echocardiography and ICA, whereas CCTA and FFRCT yielded high diagnostic sensitivity, with low specificity for CCTA. Furthermore, cardiac MRI provided a superior performance in the diagnosis of ischemia-causing CAD on both a per-patient and per-vessel basis compared with the invasive FFR reference standard. Although the diagnostic superiority of cardiac MRI over non-invasive cardiac tests has been demonstrated, it has not been implemented as a standard imaging test to determine myocardial perfusion measurements. In summary, most of these studies comparing FFRCT and other cardiac non-invasive imaging modalities have used small samples, and further large randomized multicenter studies are required.
Cost-effectiveness
The 2016 updated National Institute for Health and Care Excellence (NICE) clinical guidelines recommend CCTA as a first-line investigation for chest pain in patients without known CAD.51 The addition of FFRCT to standard CCTA can reduce the healthcare costs compared with that when combining CCTA with other non-invasive modalities for evaluating inconclusive coronary lesions, for which a functional assessment is needed.52 In 2017, the NICE medical technology guidelines proposed the use of HeartFlow FFRCT to determine the extent of CAD in patients with stable recent-onset chest pain, and concluded that FFRCT analysis could save approximately £214 per patient by reducing the need for unnecessary invasive tests and treatment.53
A substudy using the clinical data from 96 DISCOVER-FLOW patients found that a CCTA/FFRCT/ICA strategy (i.e. initial CCTA, with FFRCT for patients having ≥50% stenosis, and patients with FFRCT ≤0.80 undergoing ICA and PCI) reduced the medical costs to identify functionally significant CAD requiring invasive coronary intervention by 30% relative to an ICA/visual strategy (i.e. initial invasive angiography, with PCI for stenoses ≥50% on visual assessment; $7,674 versus $10,702), and resulted in a 12% lower clinical event rate at 1 year.52 Similarly, Kimura et al. demonstrated that the CCTA/FFRCT approach (i.e. initial CCTA with FFRCT in patients with ≥50% stenosis and ICA only in those with FFRCT ≤0.80) saved 32% in medical costs and was associated with a 19% lower cardiac event rate compared with the ICA/visual strategy at 1 year.54 Additionally, the PLATFORM investigators reported a substantially lower mean cost for an FFRCT-guided strategy than the usual invasive strategy ($8,127 versus $12,145; p<0.0001).16 For FFRCT, the quality of life had been improved over a 90-day follow-up relative to the usual non-invasive testing.16 The mean costs at 90 days and at 1 year were lower in the FFRCT cohort compared with the usual care cohort in the subgroup of patients with planned ICA (90 days: $7,343 versus $10,734, p<0.0001; 1 year: $8,127 versus $12,145, p<0.0001). In a retrospective analysis of FFRCT addition, Rajani et al. showed a per-patient saving of £200 compared with myocardial perfusion scanning in patients with a pre-test likelihood of CAD of 10–90%.55 The NICE guidelines for chest pain reported an average saving of £159 per patient using the adapted pathway with HeartFlow FFRCT, and £214 per patient for FFRCT compared with the current treatment pathway for all functional imaging tests (SPECT, MRI and echocardiography).53 Most of the PCIs and surgical revascularizations were performed following ICA, and sometimes with an invasive FFR. Given that FFRCT and invasive FFR are significantly correlated, FFRCT may facilitate the planning of revascularization strategies in individual vessels and patients.53
Future Trials
The results from future multicenter studies will determine the role of FFRCT in evaluating symptomatic patients with stable chest pain.
The FORECAST trial is a multicenter, randomized trial that will assess the clinical and economic outcomes of using FFRCT as the primary test to evaluate patients presenting with stable chest pain. A total of 1,400 patients will be randomized to receive either FFRCT or standard treatment outlined in the NICE guidelines for stable chest pain. The primary endpoint is resource utilization, and the secondary endpoints include MACE and quality of life.56
The DECIDE-Gold (Dual-energy Computed Tomography for Ischemia Determination Compared to Gold Standard Non-invasive and Invasive techniques) is a prospective, multicenter study. The diagnostic accuracy of CCTA with FFRCT is being evaluated against dual-energy CT perfusion imaging for non-invasive assessment of the hemodynamic significant coronary stenosis in 156 patients, as compared with invasive FFR as the reference standard. Additionally, the performance of FFRCT and/or dual-energy CT will be determined compared with myocardial perfusion imaging.57
Conclusion
CCTA-derived non-invasive FFR is a novel approach with a high diagnostic yield for the detection and exclusion of flow-limiting coronary lesions. It plays a vital role, especially with regard to patients referred to ICA, and bridges the gap between anatomic imaging and clinical decision-making. FFRCT correlates well with invasive FFR and provides high diagnostic accuracy and discrimination to identify hemodynamically significant CAD when compared with invasive FFR as the reference standard. FFRCT has the potential to overcome the major limitations of anatomic testing using CCTA, such as low specificity, and substantially improves the detection of obstructive CAD. Integrating FFR into CCTA not only helps to rule out obstructive CAD but also provides information on anatomic and lesion-specific stenosis to facilitate revascularization procedures. FFRCT >0.80 safely identifies patients with an excellent medium-term follow-up that could be managed with optimal medical therapy. In the next 5 years, the UK may be able to provide essential clinical experience by clinically implementing CCTA and FFRCT chest pain pathways according to the NICE guidelines. Future research should aim to collect prospective, randomized and long-term outcome data for clinical guidelines in the coming years. The class and level of recommendation will depend on the prognostic information and cost-saving benefits in future clinical trials.
Click here to view Supplementary Material.