Initially thought to be a passive degenerative process, coronary artery calcification (CAC) is commonly indicative of advanced atherosclerosis.1,2 CAC is associated with decreased vascular compliance and increases the risk for adverse cardiovascular events.3
CAC becomes more prevalent with advancing age, and above age 70 years it is more frequently encountered in men than in women (90% versus 67%).4,5 Susceptibility to CAC is also higher in those with dyslipidemia, diabetes, hypertension, obesity, and chronic kidney disease.6
With an aging population, CAC is increasingly encountered in modern-day interventional practice. Unfortunately, it is associated with lower procedural success and higher rates of periprocedural complications, such as failure to deliver stents, perforations, dissections, and other major adverse cardiac events (MACE).7 Furthermore, suboptimal stent deployment in the setting of severe calcification is associated with both short- and long-term MACE (stent thrombosis, MI, in-stent restenosis, and target lesion revascularization), thought to be because of suboptimal stent deployment and lower minimal stent areas.8
Histopathology of Coronary Artery Calcification
Intimal calcification is the predominant form of calcification seen in coronary arteries, as opposed to the medial calcification seen in peripheral artery disease. The process of CAC deposition starts with microcalcification as a result of pathological intimal thickening. Within the lipid pool, microcalcification occurs due to smooth muscle cell apoptosis and macrophage-derived matrix vesicles. Microcalcifications coalesce over time to form speckles and fragments, eventually forming sheets or plates of calcification. Fracture of calcified sheets results in nodular calcification.9 These calcified nodules may protrude into the lumen, leading to disruption of the endothelium and underlying collagen matrix resulting in coronary thrombosis, although the pathological mechanism whereby calcified nodules can lead to acute coronary syndrome account for only 2–7% of the incidence of acute coronary syndrome.10 The majority of acute coronary syndromes are because of fibroatheromas, thin cap fibroatheromas, and ruptured plaques, which tend to have large necrotic cores but minimal or no calcification. In contrast, healed ruptures or fibrocalcific plaque tend to have severe calcification – out of proportion to the necrotic core – and often present as stable coronary artery disease (CAD) with progressive luminal narrowing.11
Diagnostic Modalities
CAC is typically identified by fluoroscopy, CT coronary angiography (CTCA), intravascular ultrasound (IVUS), and/or optical coherence tomography (OCT).
Fluoroscopy
Fluoroscopic detection of CAC has been shown to have a sensitivity between 40% and 79% with a specificity between 52% and 95%.12–14 In patients with chronic total occlusions, Fujii et al. detected calcium using IVUS in 96% of patients, whereas it was only detected in 61% using fluoroscopy.15
Fluoroscopic grading of calcium during angiography is classified as follows: none or mild; moderate calcification noticed only during cardiac motion before contrast is injected; or severe if radiopacities are seen without cardiac motion, often with the characteristic tram-line calcification.
CT Coronary Angiography
Currently, CTCA is the most commonly used non-invasive tool used to directly identify CAD, which includes an assessment for CAC. The coronary artery calcium score (CACS) was first reported by Agatston et al. and now is widely used to quantify the calcium burden in coronary arterial beds.16 The score is divided into three groups: 0–100, 101–400, and >400. Budoff et al. documented that patients with a CACS of >100 had a two- to five-times higher risk of suffering an acute coronary event in near-term follow-up.17 Large-scale observational studies have also supported the role of CACS in cardiovascular risk stratification, especially in patients who are at an intermediate risk of events.18–20
Intravascular Ultrasound
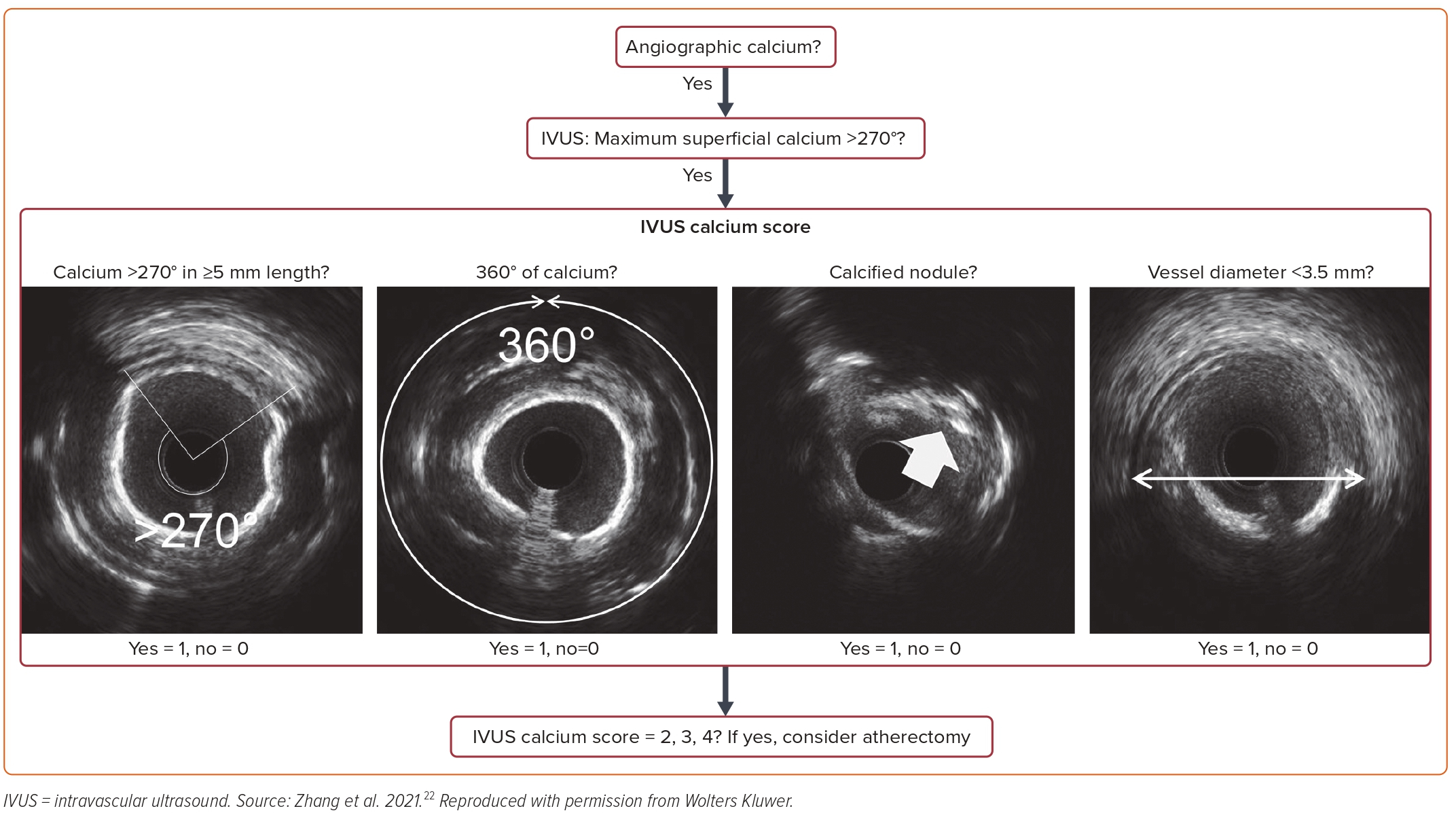
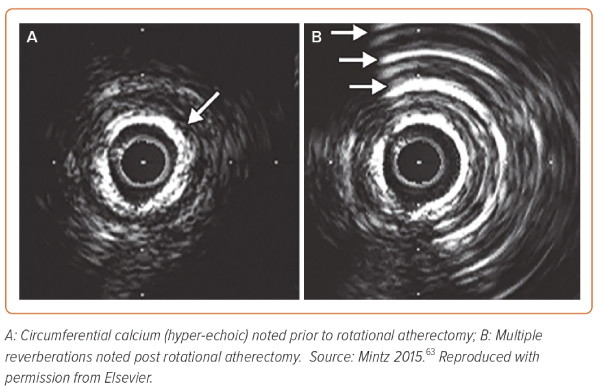
The IVUS beam is strongly reflected by calcium, resulting in a classical signature finding of hyperechoic (echo-dense) plaque that is brighter than the reference adventitia and casts a shadow over deeper arterial structures (Figure 1A). Furthermore, following plaque modification with either orbital atherectomy (OA) or rotational atherectomy (RA), the fractured calcium produces reverberations that occur because of multiple reflections from oscillations of ultrasound waves between the transducer and calcium segments, resulting in concentric arcs at reproducible distances (Figure 1B). The echo-dense plaque along with shadowing is highly sensitive and reverberations are highly specific. IVUS quantitatively assesses calcium according to the arc (measured in degrees) and length of calcium deposition. It is semi-quantitatively graded as absent or subtending into one, two, three, or four quadrants. On this basis, it is classified as: class I, 0–90°; class II, 90–180°; class III, 181–270°; and class IV, >270°. Quantitative gradation is based upon its location: superficial or deep lesion. Superficial is defined as when the leading edge of acoustic shadow lies within the shallow 50% of the plaque and media thickness, whereas deep lesion is dubbed as when the leading edge of acoustic shadow lies within deepest 50% of the plaque and media thickness.21 Furthermore, an IVUS scoring system has been developed that predicts stent under-expansion and hence identifies lesions that would benefit from plaque modification prior to stenting (Figure 2).22
Radiofrequency Intravascular Ultrasound
Presently, three radiofrequency IVUS (RF-IVUS) technologies have been validated in vitro with high sensitivity, specificity, and predictive value.23 Each of these technologies uses a different approach for tissue classification. Of these, IVUS Virtual Histology (IVUS-VH) (Volcano Corporation) is widely available.
Using a mathematical autoregression model, IVUS-VH software enables automated border contour detection by spectral analysis. After planimetry, tissue classification is performed for the intended plaque area. The four plaque components are color-coded as fibrous tissue (dark green), fibro-fatty tissue (light green), necrotic core (red), and dense calcium (white).24
The other two RF-IVUS technologies are iMAP (Boston Scientific) and integrated backscatter IVUS (Visiwave, Terumo).
Optical Coherence Tomography
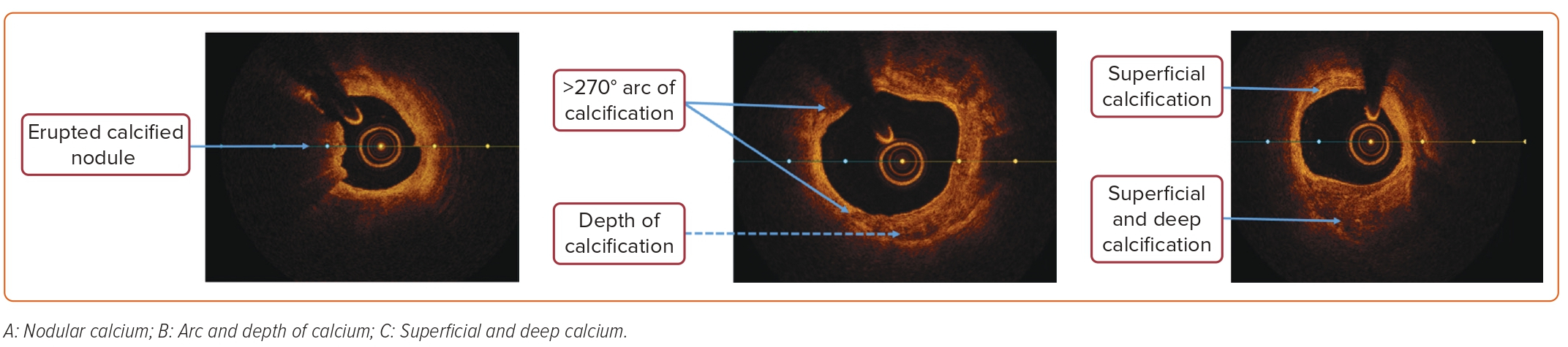
OCT has a general operative principle similar to IVUS, but it differs from the latter by using infrared light instead of ultrasound. OCT tends to produce higher resolution intracoronary images compared with IVUS, at the cost of being less penetrative. The current maximum tissue penetration with OCT is approximately 1.5–3 mm; this results in non-visualization of certain arterial structures, including external elastic lamina. IVUS, on the other hand, has a penetration of 10 mm, enabling the measurement of plaque volume. With its higher resolution, OCT fares better in greater penetration of calcium, and hence can measure its thickness, area, and volume; this makes it possible for automated quantification of these parameters (Figure 3).25,26 Table 1 highlights the differences between OCT and IVUS.
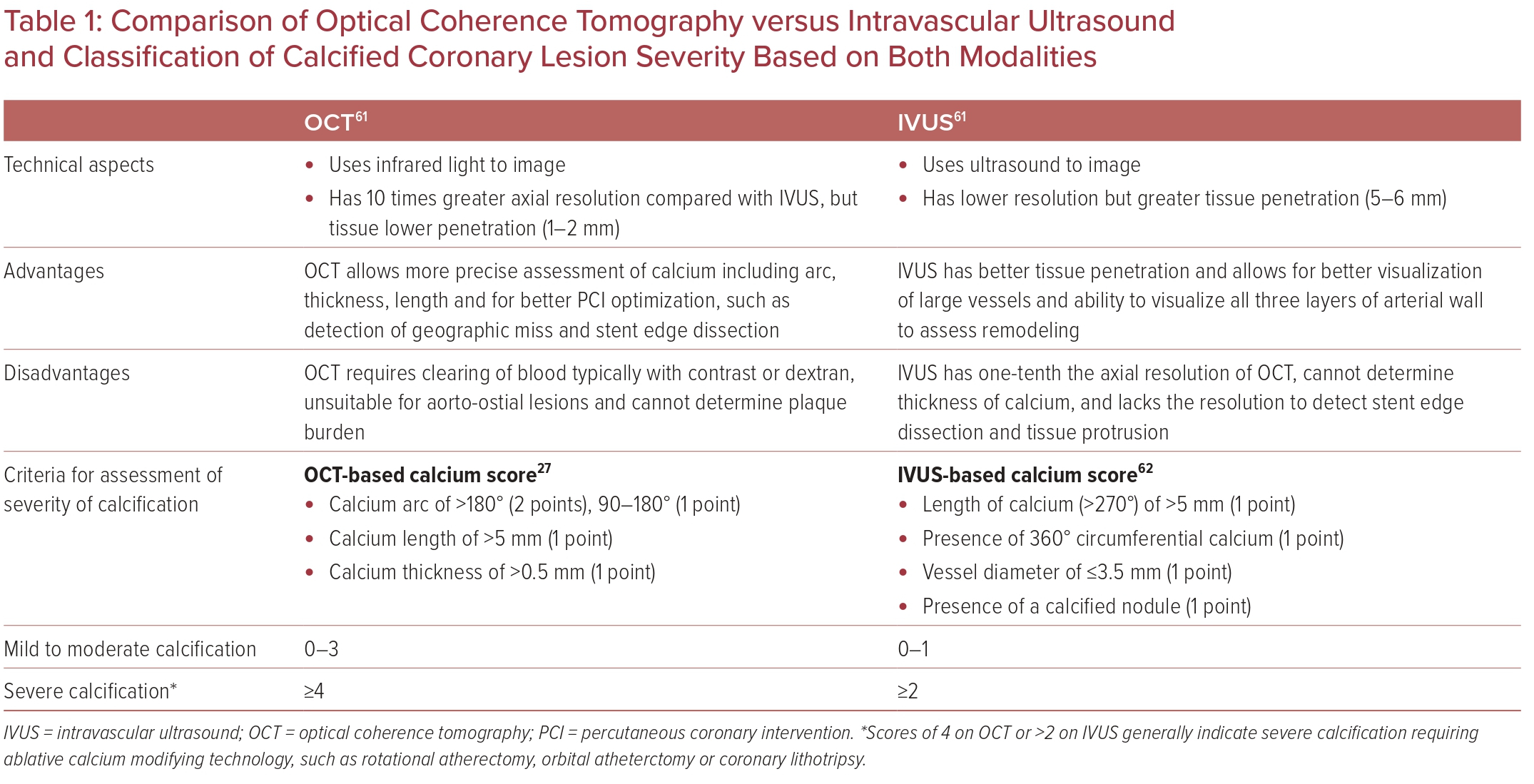
An OCT-based calcium scoring system has also been developed, which helps identify CAC lesions that are at risk of stent under-expansion and would benefit from plaque modification prior to stent implantation. It is based on three parameters (a maximum calcium angle > or <180°; maximum calcium thickness > or <0.5 mm; and maximum calcium length > or <5 mm). With a calcium score of 4 (maximum angle >180°, maximum thickness >0.5 mm, and length >5 mm), there is a greater risk of stent under-expansion (Table 1), which would require plaque modification prior to percutaneous coronary intervention (PCI).27
Treatment Modalities
In the presence of severe CAC, stent expansion is impeded and associated with adverse events such as stent thrombosis and restenosis.26 In a meta-analysis of 16 randomized control trials (RCTs), PCI with severe CAC was associated with higher mortality, higher rates of MI, and less frequent complete revascularization.28 Thus, severe CAC appears to be an independent predictor of poor prognosis.
The challenges of treating CAC have been addressed by various calcium-modification tools over the past two decades. These approaches work to ablate or fracture calcified plaques, leading to improved procedural success and improved stent expansion with larger minimal stent areas.
Balloon Angioplasty
The use of plain balloon catheters for dilatation of severe CAC lesions increases the likelihood of procedural failure and complications.29 The varying amount of calcification across the extent of the lesion causes nonuniform force application of balloon expansion on the vessel wall, increasing the risk for dissection, MI, and MACE.30 However, balloon dilation is typically the primary modality initially chosen to modify calcified coronary lesions. Non-compliant (NC) balloons are the first choice in mild to moderate cases of CAC. In these cases, balloon angioplasty should start with a smaller-sized balloon (balloon/artery ratio <1) with inflation pressure of 8 atm gradually escalated to 16–20 atm. Stent deployment is advised against in cases of lesion non-expansion.
In severe CAC, where the use of NC balloons as described above have failed to result in full balloon expansion, a double-layered OPN NC balloon (SIS Medical) can be used. These balloons can be inflated up to 40 atm, where a success rate of 75% has been reported in calcific non-dilatable lesions without significant adverse events.31 These OPN balloons have provided a new path for dilating calcific lesions as well as for under-expanded stents where other NC balloons were not able to produce desirable results. However, they are still limited by severe calcification and work best in cases where the calcium arc is <200° and calcium thickness is <6 mm.32
Modified Balloons (Cutting and Scoring Balloons)
Both cutting and scoring balloons can also be used to treat calcified coronary lesions. Cutting balloons (Boston Scientific) have three to four sharp metal microtome blades, which are mounted on a NC balloon that incises and scores the atheroma during inflation. The principle of these balloons is that they improve compliance of vessel by creating discreet lesions in the plaque, thereby ensuring controlled dissection, reducing the chance of recoil and allowing for greater expansion of the lesion.33,34 However, they do not remove calcium from the vessel wall. Cutting balloons are indicated in relatively short lesion lengths (20 mm), and the pressure of the balloon should not exceed beyond 12–14 atm to prevent embedding of blades into the vessel wall. However, these balloons are not recommended in class III–IV lesions as defined on intravascular imaging.35
The AngioSculpt Scoring balloon (AngioScore) is considered as an alternative to the cutting balloon. It contains a flexible nitinol scoring ribbon with three spiral struts that incise plaque on inflation. As it has a low crossing profile, this system is encouraged as a more flexible alternative to cutting balloons; however, no RCTs of the device have been reported.36
Another example of modified balloons is the Chocolate XD balloon (Teleflex). The balloon’s proprietary nitinol-constraining structure creates ‘pillows’ and ‘grooves’, which are designed to provide predictable, uniform, and atraumatic dilatation. Nevertheless, published data on its use in calcified CAD are limited (Supplementary Table 1).
Rotational Atherectomy
Lesions that cannot be crossed or expanded with conventional balloon angioplasty qualify for atherectomy.37 Based on differential cutting theory, rotational ablation tends to preferentially engage the rigid atheromatous plaque that is not able to deflect it, yet it preserves the integrity of the more flexible noncalcified vessel wall. The abraded plaque is degraded into smaller particles (<10 μm in diameter) and eventually cleared up by the reticuloendothelial system.38 The optimal burr size in comparison to the reference vessel diameter is 60–70%. When compared with aggressive burr sizing (burr : artery ratio >0.7), the use of smaller burr sizes diminishes angiographic complications and peri-procedural enzymatic leaks.39
RA alone has been associated with increased neo-intimal hyperplasia, and the need for repeat revascularization attributed to platelet activation and thermal injury.40,41 Current thin-strut drug eluting stent implantation after RA achieves a superior long-term outcomes.42 In the PREPARE-CALC trial, RA was compared with balloon angioplasty with modified balloons in highly calcified lesions and was demonstrated to be superior to balloon angioplasty with higher procedural success and superior stent expansion (Supplementary Table 1).43
Certain precautions are very important while using RA. The rotating burr should never be advanced close to the spring tip of the Rotawire as it can shear the spring tip off the distal end of the wire. The rotating burr should also not be allowed to remain stationary at one location in the artery; rather a gentle retraction and re-advancement (‘pecking’ movement) is needed to prevent dissection and welding.
Orbital Atherectomy
OA has a slightly different mechanism of action based on the principle of elliptical burr movement of the diamond-coated crown.44 The orbital diameter of the crown motion expands radially via centrifugal force while its elliptical orbit may allow blood flow around the crown during treatment. This theoretically helps in dispersing heat more efficiently than RA.45 Furthermore, unlike RA, which comes with different burr sizes (1.25, 1.5, 1.75, 2.0, 2.25, 2.5 mm), OA comes in one size – a 1.25 mm crown. The atheroablative lumen size obtained depends on the chosen rotational speed and speed of crown advancement, with higher speeds and slower advancement generating larger ablative areas.44,45
The limited randomized data that compare RA and OA indicate that both modalities are comparable regarding both safety and efficacy, and the choice is down to operator preference and device availability (Supplementary Table 1).46
Laser Atherectomy
The mechanism of action of laser atherectomy involves a thermomechanical process. Ultraviolet B radiation is generated by the laser, which is then absorbed by protein and nucleic acid that transfers heat to water. This results in vaporizing intracellular water, thereby explosively lysing the cells along with generating stress waves. The resulting barotrauma is exploited to treat rigid and non-dilatable calcific coronary lesions.
Several RCTs have been conducted comparing the use of pulse wave laser with other currently available modalities, but none have shown benefit over conventional percutaneous transluminal coronary angioplasty (Supplementary Table 1).47–49
Currently laser angioplasty has been recommended in seven types of lesions: long lesions, moderately calcific lesions, in-stent restenosis, saphenous venous graft lesions, ostial lesions, total occlusion, and non-dilatable lesions (some interventionalists reserve its use only for non-dilatable/non-crossable lesions).50
Intravascular Lithotripsy
Intravascular lithotripsy (IVL) is a novel technique that has evolved from similar technology used for the treatment of ureterorenal calculi. This technology has been adapted for use in calcified arterial lesions with the Shockwave IVL catheter (Shockwave Medical).51 This device is a single-use, disposable catheter on which there are multiple spark gap-based lithotripsy emitters along the shaft of an integrated balloon. The balloon (sized 1:1 to vessel diameter), is filled with a 50:50 saline/contrast mixture (as ions are required for the generation of sparks), and is inflated to sub-nominal pressure (4 atm), which is enough to provide apposition with vessel wall. This establishes an effective fluid–tissue interface, which facilitates efficient transmission of an acoustic pressure wave (1 pulse/second) that travels circumferentially through the vessel wall, inducing both superficial and deep calcium plaque fractures. This results in enhanced vessel compliance, reduced fibroelastic recoil, and facilitation of stent expansion and luminal gain.52
Compared with balloon-based technologies (high-pressure NC and cutting/scoring balloons), IVL has the advantage of using acoustic shockwaves delivered by semi-compliant balloons at a sub nominal pressure, thereby avoiding high-pressure balloon-induced barotrauma. Athero-ablative technologies (RA or OA) have their own limitations, which include limited therapy to deeper calcium, thermal injury, and a higher risk of vascular complications. Compared with other options, IVL minimizes the risk for vascular complications and results in fractures in both superficial and deep calcium.
Initially approved for peripheral vascular applications in 2016, IVL gained Food and Drug Administration approval for coronary application in 2021 based on the DISRUPT II and III trials.53–55 The safety and effectiveness of IVL have been reported across multiple clinical studies wherein severely calcified coronary and peripheral arteries were treated, and IVL now plays an important role in current clinical practice (Supplementary Table 1).56,57
Treatment Algorithms
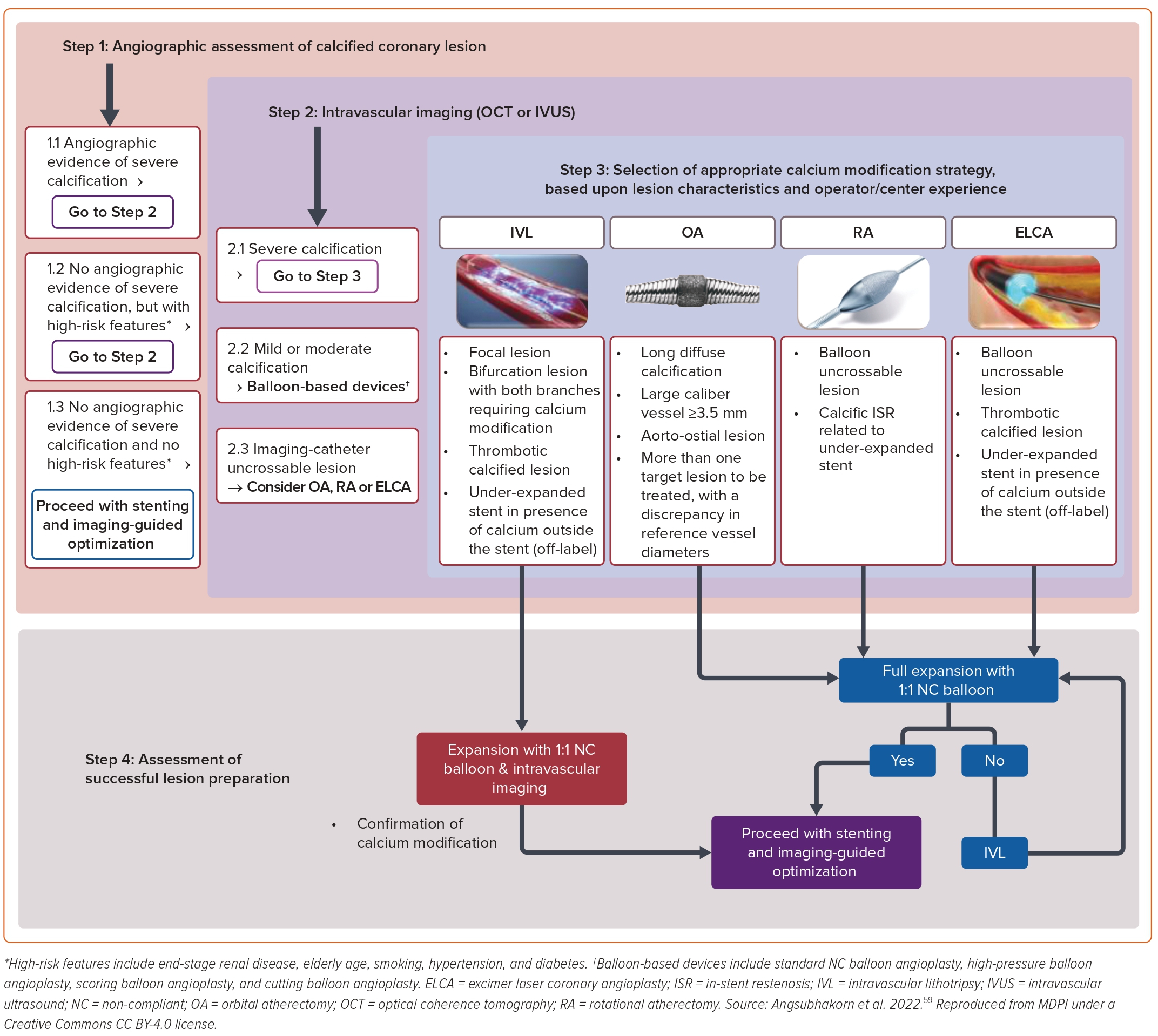
The 2021 American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions guidelines recommend using intracoronary imaging for procedural guidance in complex coronary intervention, which includes calcified coronary lesions (class 2a). Several treatment algorithms have been proposed that use intracoronary imaging to guide treatment of calcified lesions.58,59 The key determinants in most such algorithms are the extent of the calcium arc, the depth and length of the calcified plaque, and the minimal luminal area. All these parameters can only be appreciated if intravascular imaging is performed. Inability to pass an imaging catheter frequently mandates athero-ablative technologies such as RA or OA. While these ablative therapies may be sufficient to adequately modify the plaque, adequate plaque modification (calcium fractures) should be verified by subsequent intracoronary imaging. Figure 4 is an example of such a proposed treatment algorithm.60
Conclusion
Patients with significantly calcified coronary lesions pose considerable challenges during percutaneous revascularization. Treatment of these lesions is associated with increased periprocedural MACE rates compared with non-calcified lesions. However, the identification of these lesions is key, as it allows the operator to choose a calcium-modification strategy that may mitigate this complexity prior to stent implantation. A variety of treatment options for these lesions exist, including specialized balloons, atherectomy, and IVL. While there is currently no universally accepted algorithm for choosing between treatment strategies, several different algorithms exist and the optimization of these treatment regimens will continue to evolve in the coming years.