Despite the availability of advanced medical management strategies, mechanical circulatory support (MCS), and culprit-vessel revascularization; cardiogenic shock (CS) continues to be associated with increased morbidity and mortality rates with an estimated 30-day mortality rate of 50%.1,2 Outcomes with CS have not improved over the previous three decades; possibly related to the heterogeneity of shock populations, which can make findings of clinical and retrospective trials difficult to apply globally.1,2 In this article, we will review selected major prognostication and risk scoring models for CS and discuss the recently published Society for Cardiac Angiography and Interventions (SCAI) CS classification schema.
Cardiogenic Shock: Definitions, Hemodynamic Criteria, and Clinical Indicators
CS is a clinical syndrome associated with reduced cardiac output and a secondary reduction in end-organ perfusion.3 The landmark SHOCK trial defined CS in the following way: hemodynamic indicators of CS included persistent hypotension (systolic blood pressure <90 mmHg, or the addition of a vasopressor to maintain systolic pressure >90 mmHg), reduced cardiac output (CO) (<1.8 l/min/m2 without support or 2.0–2.2 l/min/m2 with support), and concurrent elevation in left ventricular end diastolic pressure.3 Clinical signs of organ hypoperfusion from CS included cold extremities, reduced urine output, and altered mental status.3,4 Until recently, the stages of CS had not been well defined, though it was widely accepted patients requiring one or more inotropes had higher mortality.4 The topic of assessing risk in the setting of CS is quite broad and well covered elsewhere.5–7 The following are selected works but do not include all prior work.
Hemodynamic Correlate of Mortality: Cardiac Power Output
An analysis from the original SHOCK trial demonstrated that cardiac power output (product of CO and mean arterial blood pressure divided by 451) is a potent predictor of outcome.3,8 The investigators from the Detroit Cardiogenic Shock Initiative have built upon this finding in the use of the Impella microaxial flow pump to address the hemodynamic disturbance of acute MI (AMI) shock.9,10 Using a protocolized approach to shock recognition and management with percutaneous MCS, they have demonstrated improved outcomes in multicenter findings, particularly for patients who achieve an increase in cardiac power output with MCS.
IABP-SHOCK II Trial Study and Risk Score
The Intra-Aortic Balloon Pump in CS (IABP-SHOCK II) score was developed from the data set of the multicenter, open label, randomized IABP-SHOCK II trial.11,12 In this study, patients with AMI (with or without ST elevation), CS, and planned early revascularization (n=600), were randomized in a 1:1 ratio to IABP use versus no IABP use. The trial failed to show a difference in mortality between the groups. Using a stepwise multivariable Cox proportional hazards regression analysis, variables from this database significantly related to 30-day mortality (p<0.1) were identified and used as score parameters.13 These parameters included age >73 years; prior stroke; glucose at admission >10.6 mmol/l (191 mg/dl); creatinine at admission >132.6 μmol/l (1.5 mg/dl); Thrombolysis in MI flow grade <3 after percutaneous coronary intervention; and arterial blood lactate at admission >5 mmol/l. Level of risk for 30-day mortality was calculated by assigning either 1 or 2 points to each variable, leading to a score in three risk categories: low (0–2); intermediate (3 or 4); and high (5–9).13 In this sub-study, the observed 30-day mortality rates were 23.8%, 49.2%, and 76.6%, respectively (p<0.0001). Validation in the IABP-SHOCK II registry population (patients not randomized, but seen contemporaneously with the trial) showed good discrimination, with an area under the curve (AUC) of 0.79.
CardShock Trial and Risk Score
The CardShock risk score was developed from the multicenter, prospective, observational CardShock study conducted between 2010 and 2012.14 In this study, patients with CS from either acute coronary syndrome (ACS) or non-ACS etiologies were enrolled within 6 hours from detection of severe hypotension with clinical signs of hypoperfusion and/or serum lactate >2 mmol/l despite fluid resuscitation (n=219; mean age 67; 74% men).14 Data on clinical presentation, management, and biochemical variables were compared between different etiologies of shock to help stratify risk of short-term mortality. The CardShock risk score used seven variables that were associated with increased mortality: ACS etiology; age; previous MI; prior coronary artery bypass; confusion; low left ventricular ejection fraction, and blood lactate levels. The CardShock risk score was subsequently shown to predict in-hospital mortality as well (AUC 0.85).
Comparison of Risk Prediction Models
Two large studies have directly compared the prognostic accuracy of the IABP-SHOCK II and CardShock risk scores in real-world populations of patients with CS.15,16
Miller et al. looked at patients admitted to intensive care units (ICUs) in Alberta, Canada in 2015, focusing on 510 patients with CS from a registry of 3,021 patients.15 They applied the Acute Physiology and Chronic Health Evaluation-II (APACHE-II), CardShock, IABP-SHOCK II, and Sepsis-related Organ Failure Assessment (SOFA) risk scores to each patient. Scores were employed to predict in-hospital mortality with the following AUC values: APACHE-II AUC 0.72, (95% CI [0.66–0.76]), IABP-SHOCK II AUC 0.73 (95% CI [0.68–0.77]), and CardShock AUC 0.76 (95% CI [0.72–0.81]). The SOFA score was associated with an AUC of 0.76 (95% CI [0.72–0.81]).
Rivas-Lasarte et al. recently reported from the Red-Shock registry.16 Five tertiary Spanish centers collected data on all consecutive adult patients who were admitted to ICU with CS (n=696). The registry included patients with shock due to ACS as well as non-ischemic etiologies (referred to as heart failure shock). The authors applied the IABP-SHOCK II score and the CardShock score to each case. For non-ischemics, the Thrombolysis in MI (TIMI) grade needed for the IABP score was assumed to be normal flow. They found that the AUC was 0.742 for the CardShock versus 0.752 for IABP-SHOCK II, and there was no difference in the predictive accuracy between scores in their population (p=0.65).
The team who performed the IABP-SHOCK II trial and the CULPRIT-SHOCK trial have applied sophisticated modeling to a selection of 58 candidate biomarkers from sub-studies of each of the two trials.17 Using the Least Absolute Shrinkage and Selection Operator (LASSO) regression analysis technique, the group selected four biomarkers which in combination are predictive of 30-day mortality in CS patients. The components are cystatin C, lactate, interleukin-6, and N-terminal pro-B-type natriuretic peptide (CLIP). The AUC was 0.82 (95% CI [0.78–0.86]) which was superior to CardShock or the IABP-SHOCK II score. Of note, the predictive value of the score was heavily driven by lactate. Other groups have applied the CLIP combination to a broad ICU population (including sepsis and non-cardiac disease) and the biomarker-based score also seems to be predictive of survival in these settings.18
Risk scores are still more useful in research than broad clinical use outside specialized centers. This set the stage for an alternative way to classify CS that would allow clinicians across the spectrum of care to assign categories to the information available, which is usually incomplete in real-world critical care settings.
SCAI Cardiogenic Shock Consensus Statement and Classification Scheme
The SCAI assembled a multidisciplinary group to address the need for a classification of CS and subsequently the SCAI clinical expert consensus statement on the classification of CS was published in 2019.19 An additional emphasis was placed upon the use of available clinical and hemodynamic data across the continuum of care, with the goal of creating criteria that delineated the stages of CS. This new schema of CS classification was also intended for use with patients regardless of ischemic or non-ischemic etiologies and to be usable across the care spectrum from pre-hospital and emergency department providers to the catheterization laboratory and ICU. Further, inherent in this novel classification scheme was the facilitation of communication between all members of a treatment team. In addition, it was hoped that better classification of shock severity would allow therapeutic approaches to be examined across SCAI shock stages in an effort to improve the mortality rates for CS.
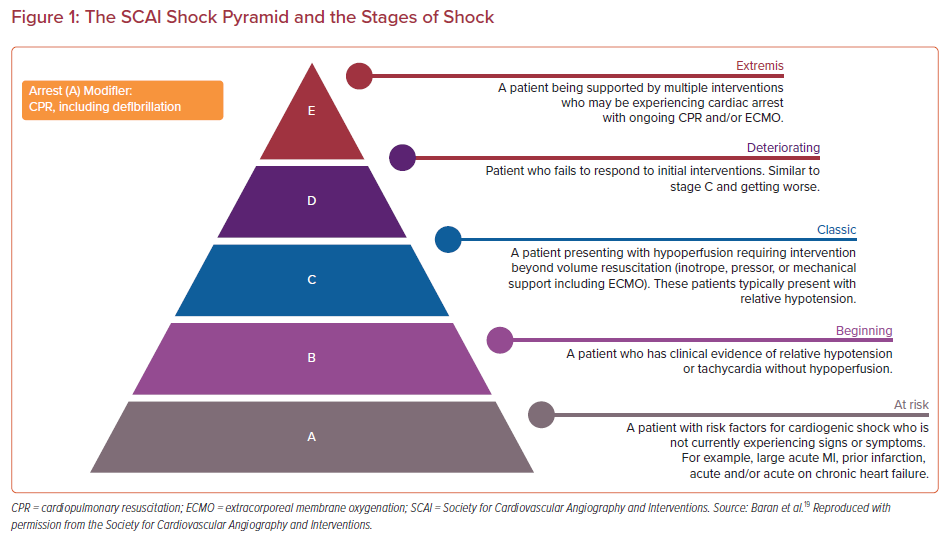
SCAI Cardiogenic Shock Stages
The expert consensus document was endorsed by the American College of Cardiology, the American Heart Association, the Society of Critical Care Medicine, and the Society of Thoracic Surgeons.19 There are five stages (A–E), with each increasing stage indicative of progressive deterioration in the patient’s clinical and hemodynamic status (Figure 1). The criteria are also meant to highlight changes in the patient’s clinical status for providers across the spectrum of care. The staging system was developed without any preceding evidence that it would accurately predict outcomes or prove to be valid. Given the broad multidisciplinary representation, there was great hope that others would validate and examine the staging system and it might lead to improvements in the design of future trials.19
Fortunately, perhaps owing to its simplicity and the lack of similar classification constructs, the SCAI shock classification has been examined quickly, and there have been several large retrospective analyses and one prospective study since its original publication in 2019.20–29
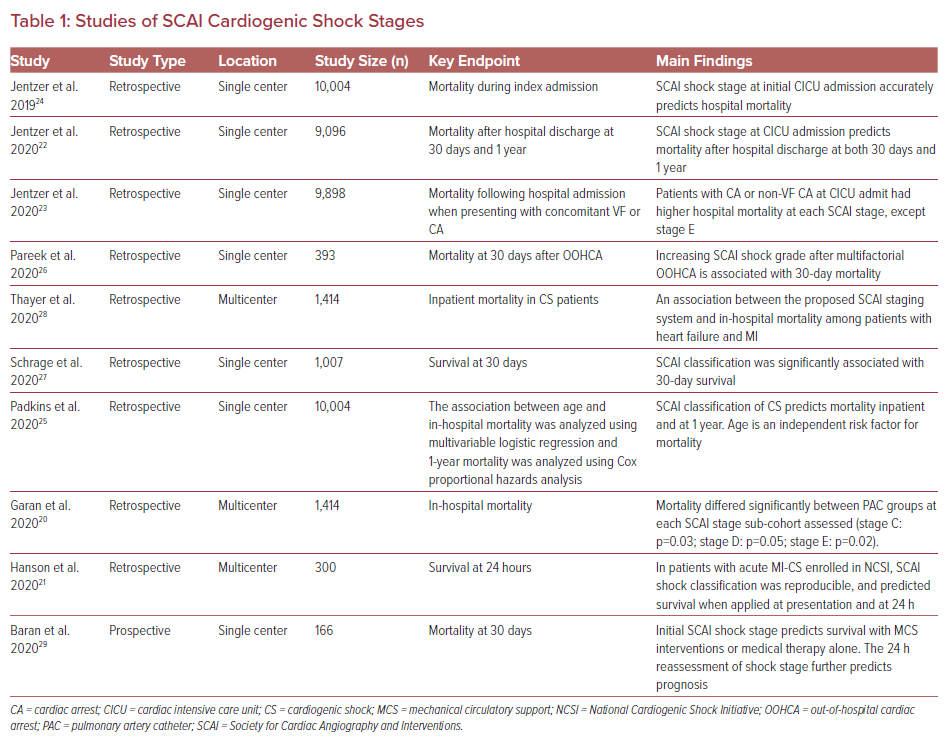
The remainder of this review will focus on a summary of validation studies, assessments of feasibility, timing of early interventions, and a discussion of future directions of the SCAI shock classification. Table 1 summarizes the key features of the papers.
Validation of SCAI Shock Stage Schema
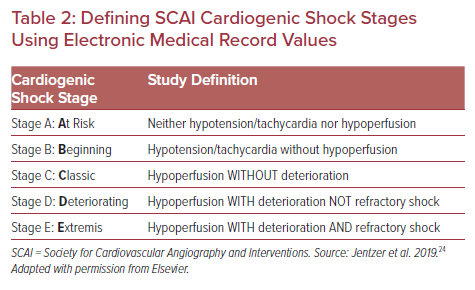
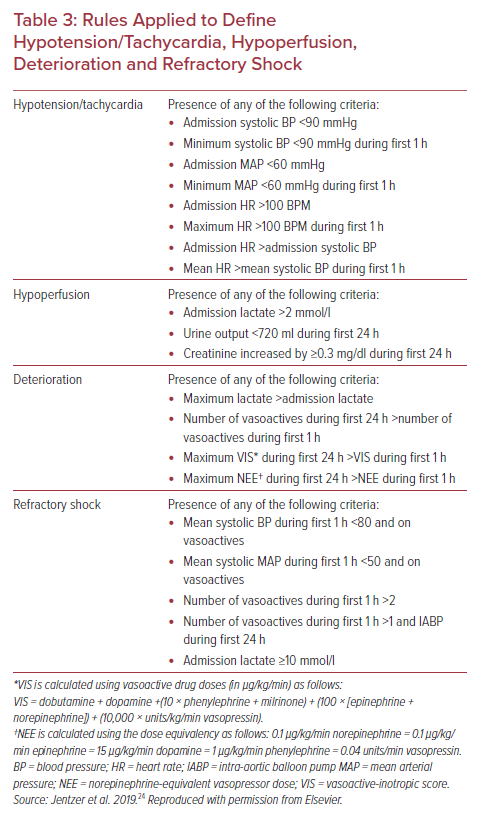
The SCAI CS staging criteria have been applied and validated in several large retrospective clinical cohorts.20–28 The first published validation involved a cohort of more than 10,000 patients admitted to a single cardiac ICU over an 8-year period. Patients were classified into SCAI CS stages A–E based on predefined vital sign and laboratory values.24 The authors set rules for translation of the SCAI shock classification into parameters that could be done by the computer or an electronic medical record (EMR). Table 2 summarizes the combinations of hypotension/tachycardia, hypoperfusion, deterioration, and refractory shock that define the SCAI stages. Table 3 shows the specific hemodynamic definitions used, which were arbitrary but set a priori by the authors.24
Each successive shock stage was associated with an increase in unadjusted cardiac ICU and in-hospital mortality rate. The proportion of patients with SCAI shock stages A, B, C, D, and E were 46%, 30%, 16%, 7%, and 1%, respectively. SCAI shock stage was predictive of outcome regardless of the underlying etiology of CS. Of the study population, 43% had ACS, 46% had heart failure (HF), and 12% had cardiac arrest.24 Additionally, each higher SCAI shock stage was associated with increased adjusted post-discharge mortality as compared to SCAI stage A (all p<0.001) and results were consistent among patients with ACS or HF.22
Further, in seeking to identify specific risk factors associated with increasing risk of mortality from CS, two separate studies employed multivariable logistic regression in this same clinical cohort. These studies demonstrated that both older age and out-of-hospital cardiac arrest were additional risk factors for mortality independent of SCAI CS stage, both during index hospitalization with CS and up to one year after.23,25
In a separate single-center study involving a cohort of 1,007 CS patients (ACS or non-ischemic shock), the SCAI classification was applied retrospectively looking at mortality.27 In this study, logistic regression demonstrated that a higher SCAI classification was significantly associated with lower 30-day survival (p<0.01); where survival probability was 96.4% (95% CI [93.7–99.0%]) in stage A, 66.1% (95% CI [50.2–87.1%]) in stage B, 46.1% (95% CI [40.6–52.4%]) in stage C, 33.1% (95% CI [26.6–41.1%]) in stage D, and 22.6% (95% CI [17.1–30.0%]) in stage E.
The SCAI stages were also applied retrospectively to a subset of patients in the National Cardiogenic Shock Initiative database (NCSI). The NCSI is a multicenter, single arm prospective registry study which seeks to assess outcomes with early implementation of the Impella percutaneous MCS device and the use of invasive assessments for guiding shock management.21 With a sample size of 300 patients, SCAI shock stage was assigned retrospectively by two independent practitioners to patients who presented with AMI CS and needed MCS therapy. Patients were categorized as SCAI stages C–E in the first 24 hours after an initial CS diagnosis; survival to hospital discharge was seen in 76% of patients assigned to stage C or D, compared to those in stage E (58%; p=0.006). Additionally, in patients who were initially assigned a lower SCAI stage (C or D) and who deteriorated to stage E over 24 hours, <20% survived to hospital discharge. In addition to further validating the use of the SCAI CS system, this study also showed that this schema of classifications could be applied with little inter-observer variability although it was done retrospectively.21
CS may result from many causes, and previous classification schemes have failed to adequately characterize discrete shock stages that could be used regardless of etiology. When the SCAI shock criteria were applied to a 5-year observational registry of 393 patients with CS in 2012–2017 following an out-of-hospital cardiac arrest (OHCA) of any cause, a stepwise and statistically significant increase in 30-day mortality was noted as shock stage increased (A: 28.9% versus B: 33.0% versus C: 54.5% versus D: 59.3% versus E: 82.9%; p<0.0001).26 There were 107 (27.2%) patients assigned to stage A, 94 (23.9%) in stage B, 66 (16.8%) in stage C, 91 (23.2%) in stage D, and 35 (8.9%) in stage E.10 Escalating SCAI shock stage was also associated with the need for renal replacement therapy, and death from multiorgan failure in addition to that resulting from cardiac causes.26
The Cardiogenic Shock Working Group (CSWG) reported on 1,414 CS patients with SCAI shock stage (assessed retrospectively) along with pulmonary artery catheter (PAC) data. The study cohort consisted of patients seen in 2016–2019 and were grouped into those with and without PACs placed prior to initiating MCS.20 PAC data was available for 598 (42%) patients and temporary MCS devices were used in 1,190 (84%) of those patients. SCAI stage predicted mortality as in other studies. The PAC assessment group had the lowest in-hospital mortality across all SCAI stages. Patients without a PAC had a higher in-hospital mortality (adjusted OR: 1.57; 95% CI [1.06–2.33]).20
In a separate study from the same registry, SCAI stages were applied to assess the risk of in-hospital mortality in patients with decompensated HF or MI.28 Risk for in-hospital mortality was associated with increasing SCAI stage (OR 95%; CI 3.25 [2.63–4.02]) in both MI patients (who made up 35% of the study registry) and decompensated HF cohorts (50% of the registry). Additionally, hemodynamic data was available in 1,116 patients (79%) in the registry, and while elevated biventricular filling pressures were common among patients with CS, elevated right atrial pressures were specifically associated with increased mortality and higher SCAI stage.28
The SCAI shock classification was also validated prospectively in a recent single center report.29 In this study, 166 patients were evaluated by a multidisciplinary shock team over the course of an 18-month period. The team could decide to implement MCS or defer as well as optimizing medical therapy. Importantly, the SCAI stage was assessed and recorded prospectively. Initial SCAI stage was highly predictive of survival, second only to the patient’s age. 30-day survival rates were 100%, 65.4%, 44.2%, and 60% for patients with initial SCAI stage B, C, D, and E, respectively (p=0.0004). Type of MCS or lack of such was not a predictor of outcome. SCAI stage at 24 hours was also examined and change in SCAI stage was highly predictive of outcome. The best survival was noted for patients with an improvement in SCAI stage of three to four categories, but any improvement was better than either remaining at the same SCAI stage or deteriorating. The 30-day survival rates were noted to be 100%, 96.7%, 66.9%, 21.6%, and 6.2% for patients with three to four SCAI stage improvement, two-stage improvement, one-stage improvement, no change in SCAI stage and worsening of SCAI stage respectively, following initial evaluation by the shock team (p<0.0001).29
Future Directions
The purpose of risk scores or classification systems is to allow the clinician to quickly decide on a treatment strategy when confronted with a single patient (Figure 2). The best measurements can be made repetitively and will have sufficient significance to justify their assessment. Validated tools allow clinicians to focus maximal efforts where they have the best chance of success.
Several early studies have validated the use of the SCAI shock criteria in hemodynamically unstable patients regardless of etiology of CS, laying a foundation for future clinical trials. Additionally, these criteria have provided a validated risk stratification tool that serves to facilitate communication between all providers of a patient’s care team regarding clinical and hemodynamic status. In line with this important objective and widespread use of these criteria, future use of SCAI CS classification might be considered as part of the EMR given the definitions which lend themselves to simple determination. For example, the definitions set by Jentzer et al. could potentially be adapted to an EMR (Tables 2 and 3).24 Objective clinical information available from the EMR could be used in the calculation of a daily SCAI shock stage assessment and as part of the documentation of acute clinical changes. More studies are needed that examine the feasibility of SCAI CS criteria use in communications between pre-hospital providers, emergency department staff, nursing staff, and general cardiologists.
As SCAI stages are integrated into the lexicon of clinicians’ management of CS, it will be important to remember that the classification of a patient is dynamic. Depending on the care setting and local expertise, a patient with a particular SCAI stage may be appropriate for transfer or it may be appropriate to try initial interventions and reserve transfer for patients with stagnant or worsening shock stage (which indicates a poor prognosis). Clinical trials may well be designed to capture the initial SCAI stage either as an inclusion criterion or a prospective assessment at enrollment to allow effects of therapy to be assessed across the severity of illness.
Conclusion
CS remains an area of high interest with a stable incidence and no proven modality to reduce mortality beyond initial revascularization in ischemic patients. Various risk scores and more recently a classification system have been developed to categorize patients. Hopefully by carefully and prospectively ‘phenotyping’ shock patients, we will be able to develop effective therapies to target those patients who can be saved.