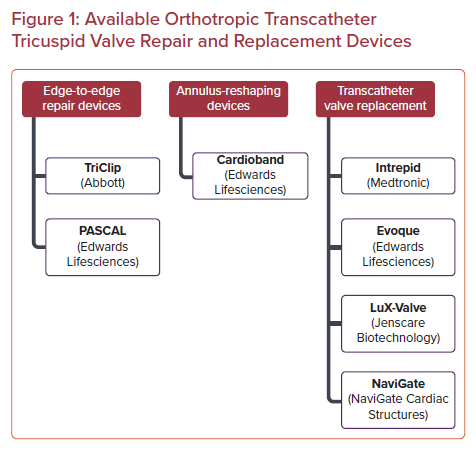
As the global population ages and grows, the burden of heart disease follows suit. In particular, tricuspid regurgitation (TR) has become a prevalent cardiac pathology, affecting more than 1.6 million people across the US and millions more across the globe.1,2 Long thought of as the ‘forgotten valve’, our understanding of tricuspid valve (TV) disease and its negative impact on survival and functional status has rapidly evolved in recent years. Numerous studies over the past decade have highlighted the increased mortality and decreased functional status that patients with severe TR experience, independent of all other comorbidities and cardiac pathologies.3,4 With an annual incidence of 200,000 patients per year coupled with a rapidly aging, more vulnerable patient population, the management of TR has rapidly evolved to meet the challenges at hand. Here, we review the current state of available orthotropic transcatheter tricuspid interventions spanning from valve repair to valve replacement for the treatment of severe TR (Figure 1).
Pathophysiology
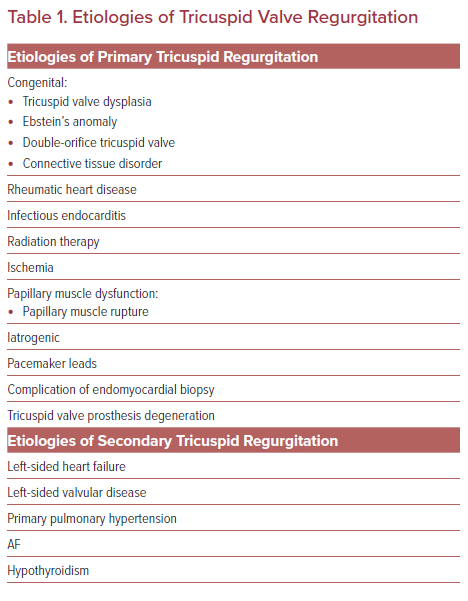
TR can be primary or secondary (functional) in etiology (Table 1). Primary TR is caused by damage to the valve leaflets and can either be congenital or acquired, such as from rheumatic heart disease, infectious endocarditis, radiation therapy, and iatrogenic in the setting of pacemaker leads or endomyocardial biopsy. Secondary (functional) TR is the most prevalent type of TR and refers to regurgitation in the setting of healthy leaflets but distorted annular geometry. Secondary TR is most often the byproduct of left-sided heart failure and left-sided valve disease with associated pulmonary hypertension. These processes lead to volume and pressure overload in the right ventricle that then undergoes remodeling with extensive right ventricular geometric deformation, chamber enlargement and annular dilatation yielding malcoaptation of the valve leaflets.5 Once significant TR is present, the abnormal volume load on the right ventricle will ultimately lead to additional anatomical deformation and regurgitant volume escalation and ‘secondary TR begets TR’.6 The degree of coaptation defect is important to consider as substantial coaptation defects can exclude potential application of certain transcatheter repair devices.
Isolated TR is a subtype of secondary TR, which occurs when there is tricuspid annular dilatation without right ventricular remodeling or dilatation. Isolated TR is most often seen in elderly patients with AF and right atrial dilatation.7 The development of RV failure associated with severe TR is a critical milestone in the disease’s natural history as this is frequently irreversible despite invasive therapy.8 Consequently, severe RV dysfunction represents an important exclusion criterion in trials evaluating percutaneous devices for severe TR. Timely intervention is key to preventing the occurrence of RV failure. Late in the course of the disease, right ventricular dilatation and associated interventricular septum displacement result in left ventricular impingement and development of left heart failure.9 The current generation of transcatheter TV repair and replacement devices have mainly been used in patients with severe functional TR. For severe primary TR, surgical intervention remains the primary intervention and Class I recommendation in patients without evidence of right heart dysfunction and in patients undergoing concomitant left-sided valve surgery.
Historical Context
Despite the presence of surgical techniques such as bicuspidization and DeVega annuloplasty, isolated surgical correction for functional TR has historically been avoided. Instead, the established historical consensus has been that functional TR will correct if left-sided valve disease is repaired.10 Early surgical data demonstrating that patients undergoing sternotomy for isolated TV repair had significant in-hospital mortality further reinforced the paradigm to avoid isolated TR intervention.11 The understanding of how severe TR affects patient survival started to change after a retrospective review by Nath et al. of more than 5,000 patients from Veterans Affairs Medical Centers. The authors showed that mortality was associated with the severity of TR and that moderate or greater TR was independently associated with increased mortality regardless of left ventricular ejection fraction or pulmonary artery pressure.4 Subsequently, over the past decade, a growing body of evidence has shown that the prevalence of severe TR in hospitalized patients is increasing, and also that severe TR is an independent predictor of poor survival.3,4,12 As transcatheter structural heart interventions for left-sided valve disorders have grown in availability, safety, and feasibility, the application of these interventions to correct severe TR has become of interest. The possibility of performing TV repair without sternotomy or cardiopulmonary bypass has ushered in a new era for TV interventions.
Guidelines for the Treatment of Tricuspid Valve Disease
Surgery remains the Class I recommendation for TV repair in patients with primary TR and tricuspid stenosis. For primary isolated severe TR, the European Society of Cardiology 2017 guidelines give a Class I recommendation for TV repair for symptomatic patients without severe RV dysfunction. The recommendations for severe functional TR are not as well defined due to the relative novelty of many of the transcatheter procedures and devices used in this population. According to the 2017 ESC guidelines on management of valvular heart disease, the only Class I indication for secondary TR repair is during a concomitant left heart surgery.13 As the role of transcatheter TV interventions for secondary TR continues to grow and more outcomes data following these procedures is generated, it is possible that future guidelines will evolve to reflect the shifting landscape of the field as has happened with aortic valve interventions.
Transcatheter Repair and Replacement Strategies
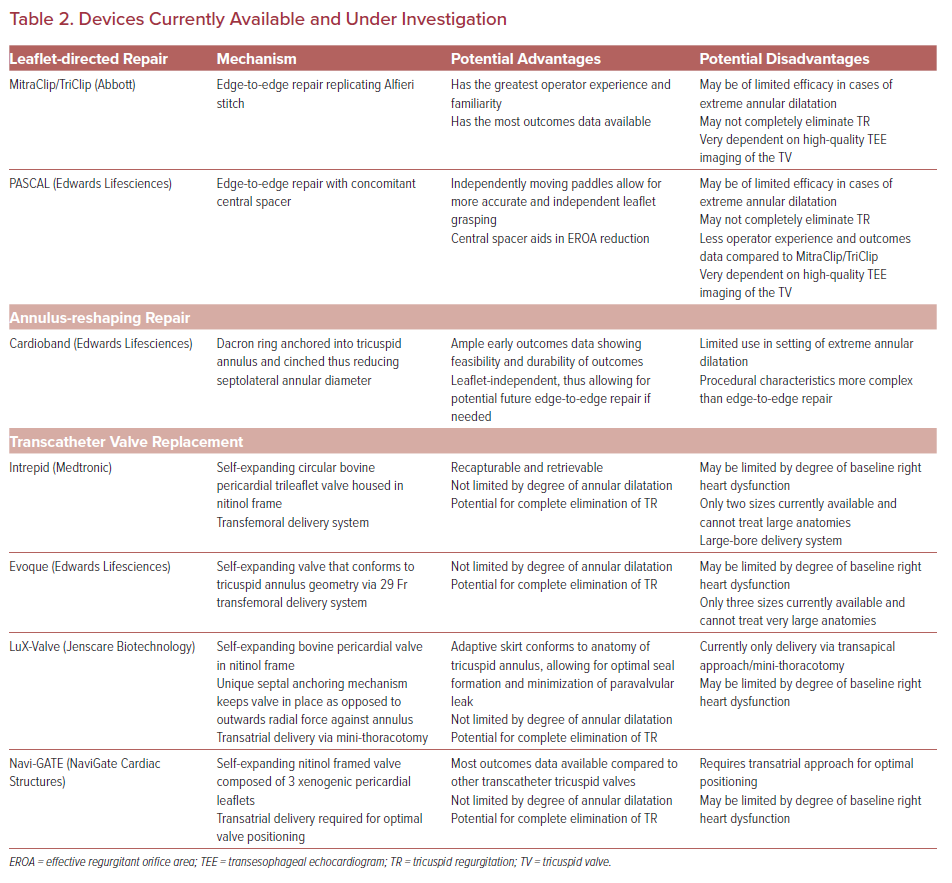
Both transcatheter valve replacement and valve repair have emerged as feasible and effective interventions for severe functional TR (Table 2). Transcatheter valve repair can broadly be grouped into two categories: leaflet-directed therapies and annulus-reshaping therapies.14 Currently leaflet-directed therapies constitute the majority of the outcomes data available, but small case series with both short and intermediate-term outcomes with annular-reshaping devices have also been published.
TV replacement is a rapidly growing area of interest with several potential advantages compared to valve repair. Valve replacement allows for complete elimination of TR, while repair often yields significant reduction but not complete elimination of regurgitant blood flow. Valve replacement can also be performed largely independent of tricuspid annulus morphology, while repair techniques are limited by the degree of annular dilation and the complexity of the underlying subvalvular apparatus.
Registry data pooling outcomes after valve repair and valve replacement have demonstrated high rates of procedural success, significant improvement in TR severity, and significant improvement in clinical symptoms at short- and mid-term follow-up.15 A closer look into the currently available transcatheter repair and replacement modalities for severe TR follows.
Leaflet-directed Therapies
Leaflet-directed therapies target the TV leaflets to reduce the effective regurgitant orifice area (EROA). They are the most widely used modality for transcatheter TV repair. Currently, three devices have outcomes data available following repair: MitraClip/TriClip (Abbott), PASCAL (Edwards Lifesciences), and FORMA Spacer (Edwards Lifesciences). The FORMA Spacer device is no longer available and will not be reviewed here.
MitraClip/TriClip
The MitraClip/TriClip system works by conceptually replicating the Alfieri stitch and was initially used for transcatheter mitral valve repair. This clip-based edge-to-edge repair technique has recently been adapted for the TV with promising early results. The EROA is reduced by attaching at least one clip to the TV leaflets. Multiple clips can be used depending on the size of the coaptation defect and the location of the regurgitant jet. The procedure is conducted via fluoroscopic and transesophageal echocardiographic guidance. As the most widely used transcatheter TR repair modality to date, operator familiarity with the procedure is a major advantage of MitraClip/TriClip.
The TRILUMINATE trial was an 85-patient prospective study looking at outcomes following TV repair with the TriClip system. At 30-day follow-up, the TR severity of 87% of patients in the study had reduced by at least one grade. Additionally, the majority of patients in the study had significant improvements in their New York Heart Association (NYHA) class and 6-minute walk distance on short-term follow-up.16 Given the promising results of the study, recruitment for the TRILUMINATE Pivotal IDE trial (NCT03904147) is now in progress. The IDE trial will recruit about 700 patients with severe TR and randomize them to medical therapy or TriClip repair. Long-term follow-up will be assessed at 5 years.
PASCAL
Another intervention originally designed for transcatheter mitral valve repair and subsequently adapted to the TV, the PASCAL repair system has shown significant promise as a leaflet-directed repair modality for TR. The PASCAL system replicates the Alfieri stitch and clipping together valve leaflets to reduce the EROA similar to the MitraClip/TriClip.
There are several distinct factors for the PASCAL system that serve as potential advantages compared with MitraClip/TriClip. First is the presence of a central spacer that sits within the regurgitant orifice, helping to reduce the EROA. Second is the ability for operators to move the device’s graspers independently and simultaneously, thus yielding greater flexibility and accuracy during the leaflet grasping process. A trial of 28 patients undergoing repair with the PASCAL system was recently published with promising results. The majority (86%) had successful device implantation with a post-procedure TR grade of moderate or less. At 30-day follow-up, 85% of patients had a TR grade of moderate or less, and 88% of patients had NYHA class I or II.17
Annular-reshaping Therapies
Annular-reshaping devices treat TR by directly reshaping the tricuspid annulus without altering the valve leaflets. A major advantage of annular-reshaping therapies is that they are ‘leaflet independent’, thus allowing operators to perform additional edge-to-edge repair if needed in the future. Annular-reshaping devices have been shown to be efficacious in decreasing TR and improving functional status in the majority of patients with severe TR; however, the utility of these devices in the setting of extreme annular dilation is limited. The three annular-reshaping devices that have been implanted in humans are Cardioband (Edwards Lifesciences), TriCinch (4Tech), and Trialign (Mitralign). However, both the Trialign and TriCinch are no longer available and will not be reviewed.
Cardioband
As with the majority of current transcatheter tricuspid repair devices, the Cardioband repair system was originally developed for transcatheter mitral valve repair. The device consists of an adjustable Dacron ring that is anchored into the tricuspid annulus without sutures. Following implantation, the ring’s diameter is reduced via a cinching mechanism, thus also reducing the septolateral annular diameter of the valve. Procedurally, both fluoroscopy and transesophageal echocardiography are used to guide ring implantation and cinching in real time.
Initial data reporting short-term outcomes following repair with the Cardioband system demonstrated high rates of procedural success and improvement in TR. In a prospective multicenter study of 22 patients with severe TR at baseline, repair with the Cardioband system yielded a 96% technical success rate and no mortality at 30-day follow-up. In this cohort, patients had 38% reduction in their EROA at 30 days. Additionally, the number of NYHA class I or II patients increased from 27% at baseline to 71% at 30-days post-procedure.18
Recently, results for the prospective Tri-Repair study (NCT02981953) were published further highlighting the technical feasibility and long-term durability of TV repair with the Cardioband system. In the Tri-Repair study, 30 patients with at least moderate TR and unacceptable high surgical risk underwent TR repair with Cardioband. Technical success was achieved in 100% of cases. At two-year follow-up, 82% of patients were NYHA class I or II compared to 17% at baseline, and 72% had a TR grade of moderate or less. Significant improvements in both the 6-minute walk distance and Kansas City Cardiomyopathy Questionnaire (KCCQ) score were also seen at follow-up. 19
Transcatheter Tricuspid Valve Replacement
Transcatheter TV replacement is a rapidly growing area of interest for the transcatheter treatment of severe TR. There are several differentiating factors – and potential advantages – that distinguish complete TV replacement from repair in the setting of severe functional TR. First, valve replacement yields complete elimination of TR whereas TV repair most often yields significant reduction but not complete elimination of TR. Second, valve replacement can be performed largely independently of TV anatomic limitations such as extreme annular dilation or complex subvalvular anatomy. In contrast, the currently available transcatheter TV repair devices are often limited in their therapeutic efficacy by pre-procedural TV characteristics such as extreme annular dilation. Last, by implanting a valve in the tricuspid annulus, transcatheter valve replacement opens the possibility for future valve-in-valve interventions if needed following initial valve replacement. There are four valves currently being investigated for use in the tricuspid position: Intrepid (Medtronic), Evoque (Edwards Lifesciences), LuX-Valve (Ningbo Jenscare Biotechnology), and NaviGate (NaviGate Cardiac Structures). A brief review of each will be presented here.
Intrepid Valve
The Intrepid valve was originally developed for transcatheter mitral valve replacement, and has recently been implemented in the tricuspid position as well. The valve is a self-expanding, circular, bovine pericardial trileaflet valve housed within a nitinol frame. A dedicated delivery system and the ability to recapture and retrieve the valve makes Intrepid different from other transcatheter options currently under investigation. Data from a recent first-in-man case series of three patients undergoing valve replacement with the Intrepid valve highlighted a 100% rate of successful valve deployment and implantation without complications.20 An early feasibility trial (NCT04433065) is currently ongoing in the US.
Evoque Valve
Available for compassionate use, with eligibility determined on a case-to-case basis per local heart team discussion, the Evoque valve is another transcatheter valve currently under investigation and recruiting for an early feasibility study in the US (NCT04221490). The valve has a unique frame that conforms to the shape of the tricuspid annulus, yielding the most optimal retention forces for each patient’s respective anatomy. To date, outcomes from 25 compassionate use cases with the 29 Fr transfemoral Evoque system have been reported. All patients had severe TR and right heart failure at baseline. Technical success was achieved in 92% of cases, and 30-day rates of mortality, stroke, device embolization, and MI were 0%. The proportion of patients with TR grade 0 increased from 0% pre-operatively to 88% after valve replacement. The number of patients with NYHA class III or IV symptoms was 24% after valve replacement, down from 96% at baseline.21 These promising results highlight the potential of transcatheter TV replacement. Future studies will continue to build on this existing literature while also evaluating long-term outcomes and valve performance.
LuX-Valve
The LuX-Valve is another nitinol-framed self-expanding bovine pericardial valve that has been used for transcatheter TV replacement. Currently, the device requires transapical deployment via a mini-thoracotomy. Unlike other valves that rely on outwards radial force against the annulus, the LuX-Valve uses a septal anchoring mechanism to remain in position. The LuX-Valve is unique in that it has an adaptive skirt which conforms to each patient’s annular anatomy, reducing the potential for paravalvular leak.
Outcomes from an early experience series of 35 compassionate-use cases were presented at the 2019 Transcatheter Cardiovascular Therapeutics Conference (TCT; San Francisco, CA). All patients had severe TR at baseline and all were either NYHA class III or IV. Transcatheter TV replacement with the LuX-Valve was successful in 100% of cases with no reported conversions to open surgery. At 30-day follow-up, mortality was 5.7%. There were no reported cases of pulmonary embolism, third-degree atrioventricular block, or right-coronary artery injury on follow-up. Significant improvements in NYHA class and 6-minute walk distance were seen after valve replacement at 30 days.22
NaviGate
The NaviGate valve is a self-expanding nitinol framed valve composed of three xenogenic pericardial leaflets. The valve is currently available in five sizes (36 mm, 40 mm, 44 mm, 48 mm, 52 mm), and requires a transapical approach for optimal positioning. Following a first-in-man case in 2016, there have been 32 compassionate-use cases of transcatheter TV replacement with the NaviGate valve worldwide. Data presented from these cases at TCT 2018 (San Diego, CA) showed that 100% of patients had either severe or torrential TR at baseline. In 100% of cases, TR was reduced to moderate or less and 96% of cases had mild TR after valve replacement. Additionally, 91% of patients were NYHA class I or II following valve replacement. The 30-day mortality rate was 12.5%. Poor baseline RV function was a reliable predictor of worse outcomes in the compassionate-use cohort.23,24 Further studies are needed to identify additional predictors of poor outcomes. The degree of RV dysfunction that should be considered limiting is also currently unknown.
Patient Selection and Current Limitations
Given the vast heterogeneity in device availability and operator experience for transcatheter TV interventions, the creation of uniformly applicable patient selection recommendations may not yet be realistic or accurate. There are, however, several considerations and uniformly applicable principles that we will highlight here. All patients with severe functional TR should be medically optimized to the fullest extent possible prior to consideration for transcatheter TV intervention. Volume management with diuretic therapy, pharmacological neurohormonal blockage, and rate control (as well as rhythm control in selected patients) should all be titrated to the maximum extent tolerated.
Left-sided valvular disease that requires intervention should be treated prior to TV intervention. The overall health and functional status of the patient should be carefully assessed and taken into consideration. Patients with a limited life-expectancy <1 year or with extreme fragility should continue with medical therapy only without invasive TV repair/replacement given the limited data on clinical benefit following transcatheter TV intervention in this population.
To evaluate patients for transcatheter TV intervention, a complete multidisciplinary heart valve team assessment should take place. Device availability and institutional experience should be factored into the decision of which modality of TV intervention to perform. Pre-procedural imaging with transthoracic echocardiography and transesophageal echocardiography should be performed in order to gain complete understanding of right ventricular anatomy and function, and of TV anatomy including the leaflets, annulus and subvalvular apparatus. Based on the device under consideration and on institutional preference, multidetector CT, cardiac MRI, and coronary angiography may also be obtained during the procedural planning phase for more detailed assessment of right ventricular structure and function and of coronary anatomy with particular attention paid to the right coronary artery.
When deciding which specific transcatheter device to select for TV intervention, head-to-head comparison and prescriptive recommendations are not yet possible given the nascency of the field and the significant regional variability present in device availability and operator experience. Generally speaking, TV replacement may be a better option in patients with TV anatomy considered suboptimal for transcatheter TV repair, such as patients with significant leaflet tethering, extreme annular dilation, and coaptation defects >10 mm. TV replacement also carries the advantage of completely eliminating TR whereas TV repair most often only reduces TR severity. The long-term benefit of complete TR elimination over TR reduction remains yet to be fully elucidated. Transcatheter TV repair may prove more beneficial when TV replacement is unable to be performed due to poor RV function, the major limiting factor for TV replacement. Deciding between the various transcatheter TV repair modalities currently available comes down to device availability and operator/institutional experience. Both annular-reshaping and leaflet-direct TV repair devices have been shown to durably reduce TR severity after repair and decrease the burden of symptoms for patients.
Unanswered Questions and Future Directions
The understanding of severe TR and its impact on patient morbidity and mortality has evolved dramatically over the past decade and the technologies and procedural capabilities available to treat severe TR have dramatically grown. As this evolution continues, the phenotype of the prototypical severe TR patient undergoing transcatheter TV intervention will likely evolve as well. Earlier detection of severe TR and earlier heart team referral prior to the onset of RV dysfunction or significant annular dilation will expand the number of potential treatment modalities operators can choose from. Heart teams will need to discover what the optimal timing for TV intervention in patients with severe TR is. Waiting too long may lead to irreversible anatomical remodeling and decreased RV function which will limit the possible treatment options. Intervening too early may lead to unnecessary risk and unclear added benefit.
The durability of both transcatheter TV repair devices and transcatheter TV valves over the long term is another unanswered question. It is possible that devices implanted within the right heart will remain mechanically intact longer than left-sided devices due to the lower pressures they are subjected to, however this hypothesis is yet to be affirmed by outcomes data due to the young state of the field. Moving forward, as transcatheter TV replacement systems evolve and experience with these systems grows, it is possible that valve replacement may become the preferred modality of intervention for severe TR due to the ability to completely eliminate it. However, whether complete TR elimination in patients with severe TR yields added benefit to post-procedural functional status and decreased mortality over TR grade reduction but not TR elimination remains unknown.
Conclusion
Valve repair and replacement play integral roles in the treatment of TR. As the number of procedures, devices, and valves under investigation continues to grow, our understanding of which interventions to use, when to use them, and for which patients will become more refined. Large patient registries such as TriValve have already been created as a collaborative means of assessing patient outcomes following TR repair. The collection and analysis of such data is essential to address the multitude of unanswered questions remaining in the field. These questions include the identification of optimal timing for valve intervention, better understanding of the influence of various anatomical considerations on device selection, and the development of a patient selection paradigm for TV repair or replacement that yields the best outcomes in each category. The forgotten valve no more, our understanding and therapeutic approach to TV disease is expanding and maturing now faster than ever. While TR remains a prevalent and globally undertreated valvular disorder, the future of TR interventions is optimistically bright.