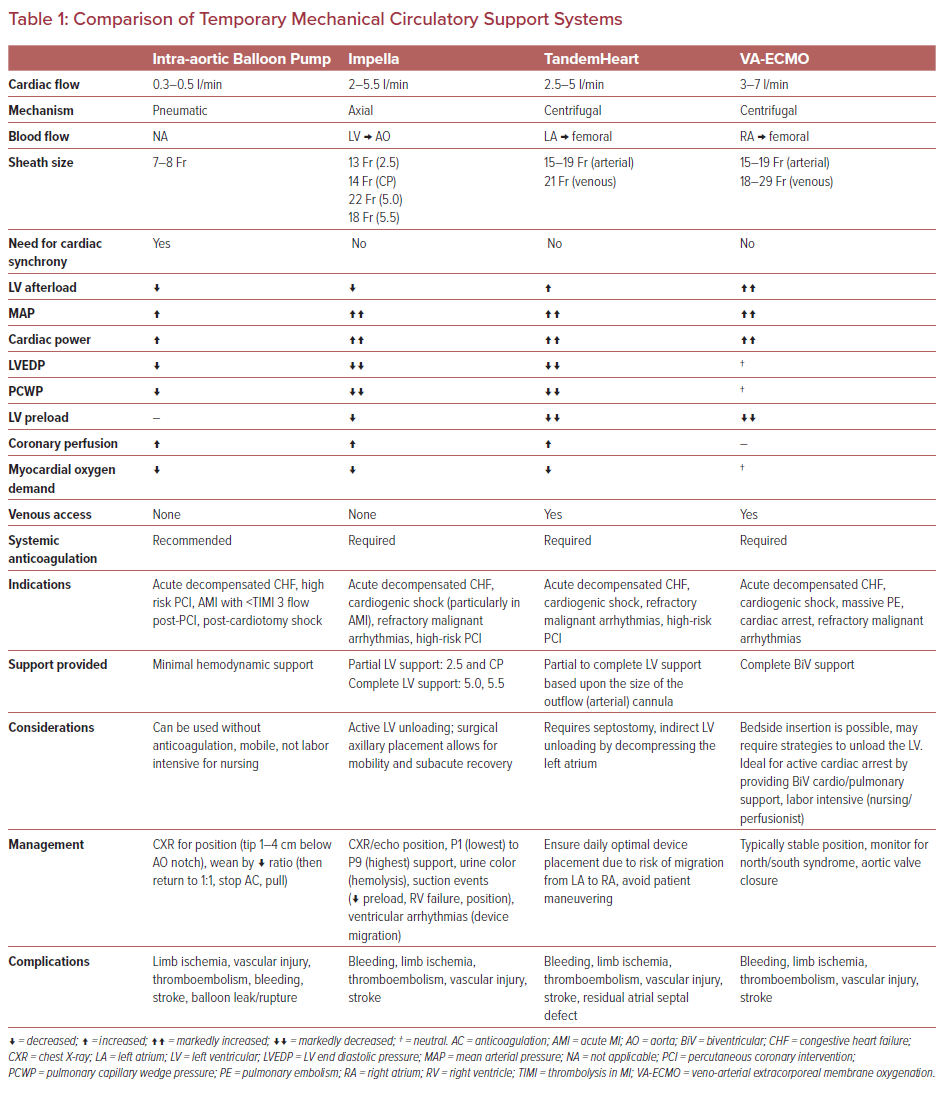
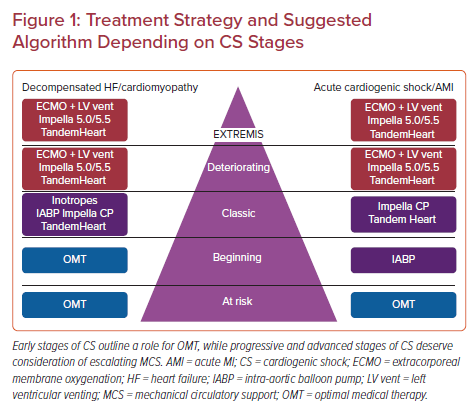
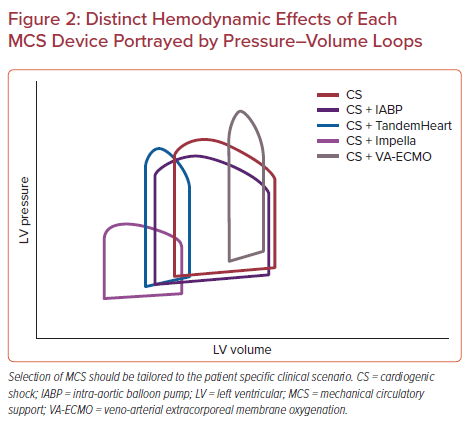
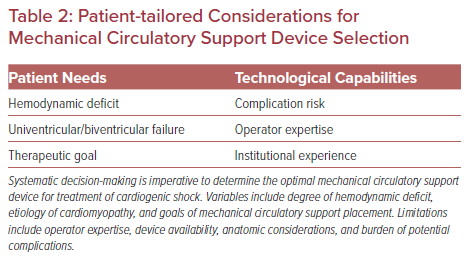
Several technologies are available to provide mechanical circulatory support (MCS) for patients in cardiogenic shock (Table 1). Device selection should be ideally customized to patients based upon the etiology of shock, the stage of shock, hemodynamic needs and the overall goal of a patient’s care (Figures 1 and 2). Furthermore, device selection must include consideration of the clinician’s and institution’s experience and expertise using specific MCS devices (Table 2).
Intra-aortic Balloon Pumps
Intra-aortic balloon pumps (IABPs) have been available since the 1960s and use counter-pulsation to provide hemodynamic support. They can be inserted from the femoral, brachial or axillary artery and are placed in the descending aorta. The balloon is inflated during diastole and rapidly deflated during systole. IABPs therefore require native cardiac contractility to function. IABPs synchronize with the heart using either a pressure or an ECG trigger for timing. There are several different IABP sizes – ranging from 40 to 60 cm3 – and they are inserted using a 7–8 Fr sheath. The device is usually set to one inflation per cardiac cycle (1:1). However, the level of support can be weaned down by changing the frequency of inflation (1:2 or 1:3). The main hemodynamic effects include an increase in coronary perfusion and decrease in afterload, which result in decreased myocardial oxygen demand and left ventricular (LV) workload.
Impella Devices
Impella (Abiomed) LV assist devices (LVAD) are continuous, axial flow devices that aspirate blood from the left ventricle and expel blood into the ascending aorta. An Impella can be inserted using a femoral, axillary or transcaval access. Currently there are four LV support systems available. The Impella 2.5 (provides ~2.5 l/min of flow) and the Impella CP (provides ~3.5 l/min of flow), are typically inserted percutaneously using a 13 Fr and 14 Fr sheath, respectively. The Impella 5.0 (provides ~5.0 l/min of flow) is typically inserted using a surgical cut down, though it can be placed percutaneously using transcaval access and requires a 22 Fr sheath. The Impella 5.5 (provides ~5.5 l/min of flow) is a shorter, more rigid device when compared to the Impella 5.0, which allows easier maneuverability in placing the device using an axillary cut down. The main hemodynamic effects of left-sided Impella devices are to increase cardiac output and cardiac power. These devices unload the left ventricle by decreasing LV end-diastolic pressure and LV wall stress. In combination, Impella devices decrease myocardial oxygen demand from LV failure.
The Impella RP is a right-sided MCS device that can be used in the setting of cardiogenic shock secondary to right ventricular (RV) failure or in conjunction to a left-sided device in patients with biventricular shock. An Impella RP must be placed from the femoral vein and requires placement using a 22 Fr sheath. An Impella RP aspirates blood from the inferior vena cava to the pulmonary artery and delivers a flow rate up to ~5.0 l/min.
TandemHeart
TandemHeart (LivaNova) is a percutaneous centrifugal LVAD that pumps blood from the left atrium to the descending aorta. The device provides flows of ~3–5 l/min based on the size of the outflow cannula. There are four main components when using a Tandem Heart device: a 21 Fr transseptal inflow cannula that is placed in the left atrium, a 15–19 Fr arterial outflow cannula, a centrifugal pump, and the control console. The cannulas are typically placed via the femoral artery and vein and require operator expertise in transseptal catheterization. The outflow cannula can be placed in the axillary artery if needed. The main hemodynamic effects of a TandemHeart device are increasing cardiac output and cardiac power. The device indirectly unloads the left ventricle by bypassing blood directly from the left atrium and therefore results in decreasing LV end diastolic pressure. Similar to the Impella, TandemHeart decreases myocardial oxygen consumption. Unlike veno-arterial extracorporeal membrane oxygenation (VA-ECMO), a TandemHeart does not require an oxygenator, as oxygenated blood is directly aspirated from the left atrium and delivered to the femoral artery via the outflow cannula.
The Protek Duo (TandemLife) is a right-sided MCS that also be used in the setting of cardiogenic shock secondary to RV failure or in conjunction to a left-sided device in patients with biventricular shock. A Protek Duo must be placed from the internal jugular vein and requires placement of a 29 Fr or 31 Fr cannula. A Protek Duo aspirates blood from the right atrium and delivers blood to the pulmonary artery using a dual lumen cannula. The Protek Duo delivers flows up to ~5.0 l/min. Unlike an Impella RP, a Protek Duo allows for the addition of an oxygenator to the circuit and therefore can function as a veno-venous extracorporeal membrane oxygenator (VV-ECMO).
Veno-arterial Extracorporeal Membrane Oxygenation
VA-ECMO provides biventricular support and oxygenation for patients in cardiogenic shock. VA-ECMO systems have five components comprising a centrifugal flow pump, membrane oxygenator, venous inflow cannula, arterial outflow cannula, and a control console. Blood is taken from the venous system typically at the level of the right atrium using a 25–29 Fr cannula placed in the femoral or internal jugular vein, oxygenated, and delivered to the systemic circulation using a 15–19 Fr outflow cannula placed typically in the femoral or axillary artery. In order to prevent limb ischemia, reperfusion sheaths can be placed in the femoral or superficial femoral arteries to improve distal limb perfusion (or in the radial, ulnar, or brachial arteries when using axillary access). VA-ECMO cannulas can also be placed using central cannulations by surgical teams to avoid peripheral complications such as limb ischemia. VA-ECMO is able to provide 4–7 l/min of flow based upon the size of the outflow cannula. Hemodynamically, VA-ECMO can increase LV afterload and end-diastolic pressure, particularly in patients with severely reduced LV ejection fractions. To overcome these physiologic concerns VA-ECMO can be combined with devices such an IABP or Impella which can unload the left ventricle. Other strategies to unload the left ventricle include septostomy or outflow cannulation placement in the left atrium (LAVA-ECMO). It is important to mention that VA-ECMO is the only device that provides biventricular support using a single device.
Goals of Mechanical Circulatory Support in Acute MI and Cardiogenic Shock
Cardiogenic shock occurs in 5–7% of patients hospitalized with ST-elevation MI but carries an in-hospital mortality rate of ~50%.1,2 Inotropes are frequently used to initially support hemodynamic collapse. The number of inotropes used, along with time on inotropic support, is correlated with increased mortality.1,2 Although inotropes aim to increase cardiac output, they also carry a risk of increased myocardial oxygen consumption and arrhythmia.3 The use of MCS to support cardiovascular collapse has therefore been increasingly used. MCS circumvents the undesirable effects of inotropes by unloading the ventricle, reducing myocardial oxygen consumption and wall tension, and increasing coronary perfusion. Use of MCS in acute MI cardiogenic shock (AMICS) may help to maintain cardiac output and end-organ perfusion, while simultaneously reducing infarct size and salvaging left ventricle function as well.4 However, these physiological benefits must be weighed against the risk of device-related complications, such as stroke, vascular access complication, bleeding, and limb ischemia. The following sections review the latest data on the use of these devices in AMICS.
Intra-aortic Balloon Pumps in Acute MI Cardiogenic Shock
Use of IABP has gradually declined after publication of the IABP-SHOCK II trial.5 Nevertheless, IABPs remain the most commonly used MCS for AMICS. The IABP-SHOCK II trial compared outcomes between use of IABP and medical therapy in those presenting with AMICS with plans for early revascularization. The study was powered to detect differences in mortality and enrolled >600 patients. All-cause mortality was similar between those treated with IABP and those in the control group at 1-month and 12-month follow-up.5 The trial led the European Society of Cardiology to downgrade its recommendation for the routine use of IABP for the management of AMICS to a class III indication, while the US guideline downgraded its recommendation to a class II indication.6 This, combined with the increased availability of other forms of MCS, has led to reduced use of IABP in AMICS.7
Use of IABP may still be considered for select patients with AMICS as a bridge to a more definitive therapy. Such examples may include patients being transferred to a shock center with more robust MCS options, in patients awaiting surgical intervention, in patients who received inadequate revascularization with <3 thrombolysis in MI flow, and in patients with significant peripheral arterial disease or inadequate vessel size for more robust MCS.
Given the lack of benefit associated with the routine use of IABP in AMICS, alternative MCS devices such as Impella, TandemHeart and VA-ECMO have become increasingly used. Nevertheless, despite this increasing use, there are no definitive studies demonstrating clear benefit or harm when using these devices. However, well-powered trials relating to the use of Impella and ECMO in AMICS are needed.
Impella in Acute MI Cardiogenic Shock
There is increasing use of Impella in AMICS in an effort to improve the physiological collapse in such patients. The Impella-EUROSHOCK registry was one of the first studies evaluating outcomes with the use of the first generation Impella 2.5 device in AMICS. The investigators evaluated outcomes in 120 patients and found that use of Impella was associated with reduced lactate levels suggesting improved systemic perfusion, but overall mortality in the study was high at 64%.8 This was followed by the results of the USpella registry, which evaluated 154 patients with AMICS. The investigators found that patients treated with Impella had better survival if they were treated with Impella pre-percutaneous coronary intervention (PCI; 65.1%) when compared to post-PCI (40.7%).9 Loehn et al. similarly demonstrated improved survival with the use of Impella before PCI (50% pre-PCI Impella versus 23.1% post-PCI Impella).10
Small, underpowered studies comparing the outcomes between Impella and IABP have not yielded definitive conclusions. The ISAR-SHOCK trial included 26 patients and demonstrated physiologic improvements with use of an Impella 2.5 compared with an IABP, with no difference in 30-day mortality.11 The IMPRESS trial recruited a very sick cohort of patients, many of whom had cardiac arrest, and is the largest randomized controlled trial comparing outcomes of Impella to IABP in AMICS.12 The study randomized 48 patients and demonstrated no differences in mortality at 1-month or 6-month follow-up.
Given the difficulties of performing randomized control trials in cardiogenic shock, matched cohort studies have also been performed.13 Schrage et al. matched patients from the IABP-SHOCK II trial to patients supported with an Impella device using a large European registry.14 The investigators demonstrated no significant difference in 30-day all-cause mortality (48.5% versus 46.4%; p=0.64), but did demonstrate higher rates of severe bleeding and vascular complications in the Impella arm. The main limitation of this study was that the degree of cardiogenic shock was not taken into account when matching patients. Helgestad et al. were able to control for the degree of shock in their matched analysis that included controlling for age, LV ejection fraction, lactate levels, kidney function, and the presence of cardiac arrest. The investigators demonstrated lower 30-day mortality in patients receiving Impella when compared to a matched control group that underwent IABP placement (40% versus 77.5%; p log rank <0.001).15
Perhaps the strongest evidence for the use of Impella in AMICS comes from the cardiogenic shock initiative.16,17 The investigators used a combination of best practices in shock management along with the use of Impella to create a standard shock protocol that was implemented in 73 hospitals across the US. Overall, the study included >400 patients with similar characteristics to patients previously enrolled in randomized control trials (i.e. the inclusion and exclusion criteria mimicked those of prior studies). The investigators found that survival to hospital discharge and at 30-days was >70%. The high survival rate was reproducible in both community and academic centers and will be the basis of a well-powered, randomized control trial.
TandemHeart Therapy for Acute MI Cardiogenic Shock
While there are several small trials evaluating the use of TandemHeart in cardiogenic shock, only a few of these studies included patients with AMICS. Thiele et al. conducted a randomized controlled study, enrolling 41 patients with AMICS. They randomized patients to receive either TandemHeart or IABP. Despite markers of hemodynamic improvement favoring TandemHeart, mortality at 30 days was similar between those treated with TandemHeart or IABP (45% and 43%, respectively).18 Burkhoff et al. similarly randomized patients to TandemHeart or IABP in patients with refractory cardiogenic shock (of the 42 patients included, 26 had AMICS). They similarly demonstrated improvements in hemodynamics including cardiac index and pulmonary capillary wedge pressure in the TandemHeart cohort, but there was no difference in mortality between the two groups.19 Finally, Negi et al. randomized 35 patients with AMICS to TandemHeart or VA-ECMO. Survival was similar in both groups (58% versus 56% at 30 days), with no difference in limb ischemia requiring surgery, need for renal replacement therapy, stroke, or recurrent MI.20
Observational studies using TandemHeart in AMICS consistently demonstrate improved hemodynamics. Kar et al. evaluated 80 patients and found that TandemHeart led to a rapid improvement several hemodynamic measures, including cardiac index, systolic blood pressure, urine output, and lactic acid levels.21 The mortality rates were 40.2% and 45.3% at 30 days and 6 months for AMICS patients. Smith et al. analyzed 55 patients, 16 (29%) of whom had AMICS, and found that survival was greatly influenced by the indication for use of TandemHeart with a survival of 23.8% when used as a bridge to recovery and 51% when used as a bridge to LVAD or surgery (p=0.04).22 They also found that patients who did not receive definitive therapy had very poor outcomes (13.8% survival to hospital discharge). Continued observational data are being collected in the THEME registry, an ongoing multicenter study (NCT02326402).
Veno-arterial Extracorporeal Membrane Oxygenation Therapy for Acute MI Cardiogenic Shock
There has been significant enthusiasm for use of ECMO in AMICS after the publication of the IABP-SHOCK study and increasing use of mobile ECMO circuits. However, similar to other MCS options, there are limited randomized studies evaluating the outcomes of ECMO in AMICS. Garan et al. conducted a prospective study in 2019 comparing the outcomes of 51 patients treated with VA-ECMO or Impella following AMICS.23 Patients with VA-ECMO required an increased number of vasopressors and were more likely to have an IABP in place when compared to patients treated Impella. Survival to discharge between the cohorts was similar (50% for VA-ECMO versus 63.6% for Impella). However, the study was limited in that sicker patients were more likely to receive VA-ECMO and 47.1% of patients were supported with both devices simultaneously, though data were analyzed according to which device was initially used.23 Vallabhajosyula et al. performed a large analysis using the National Inpatient Sample (NIS) database and evaluated ~9 million acute MI admissions from 2000–2014, of whom 4.6% were noted to have cardiogenic shock. ECMO was used in a total of 2,962 (<0.01%) patients and same-day PCI was performed in only 23% of the group. In-hospital mortality occurred in 59.2% of admissions treated with ECMO. However, the in-hospital mortality in those receiving ECMO for AMICS significantly reduced over time, from 100% in 2000 to 45.1% in 2014. A major limitation of the study was the difficulty distinguishing between the use of VV-ECMO as opposed to VA-ECMO using ICD-9 codes.24 Lemor et al. similarly used the NIS database to evaluate 6,290 admissions for AMICS who underwent PCI and were treated with ECMO or Impella. There was a strong preference for use of Impella (91%) over ECMO (9%) for hemodynamic support and a significantly higher overall in-hospital mortality for the ECMO cohort (45.5% versus 41.4%). After propensity matching, ECMO was associated with a significantly higher mortality compared with Impella (43.3% versus 26.7%). However, matching was limited as the ECMO cohort had high rates of in-hospital cardiac arrest (30% versus 16.7%), and therefore may have reflected a more critically ill population when compared to the Impella cohort.25
Sheu et al. evaluated 115 patients with AMICS from 1993–2002 without ECMO support and compared them with 219 patients with AMICS from 2002–2009 with ECMO support.26 The 30-day mortality for patients with ECMO was lower than the non-ECMO cohort (30.1% versus 41.7%; p=0.034). A subgroup analysis of patients in profound cardiogenic shock found a significant difference in mortality between groups (39.1% in ECMO versus 72% in non-ECMO; p=0.008). However, in patients without profound shock there was no significant difference in 30-day mortality between the groups (26.1% versus 21.9%; p=0.39). Esper et al. studied 18 patients who underwent VA-ECMO in the catheterization laboratory for AMICS and found an in-hospital survival rate of 67% and 6-month survival of 55%.27 More than one-third of patients had an IABP placed and were on vasopressors or inotropes. Similarly, Negi et al. studied 15 patients with AMICS (one-third presenting with cardiac arrest) and showed a 47% survival rate.28 More than 90% of patients were on one to two inotropes at the time of ECMO, 60% had an IABP, and the vascular complication rate was >50%.
Currently, there are two randomized controlled trials underway comparing the efficacy of ECMO therapy to standard treatment in AMICS (NCT03637205 and NCT03813134).
Mechanical Circulatory Support in Acute Decompensated Heart Failure and Cardiogenic Shock
The physiology of acute on chronic heart failure and cardiogenic shock (HF-CS) is distinct from AMICS and other acute pathologies. HF-CS is typically secondary to an acute decompensation in the setting of a long-standing cardiomyopathy. Decompensation can occur because of worsening valvular disease, new or refractory arrhythmias, medical or dietary non-adherence, or worsening of the patient’s underlying cardiomyopathy. Irrespective, patients with long-standing cardiomyopathies have undergone significant myocardial remodeling and compensatory physiological changes and can therefore tolerate lower cardiac outputs when compared to patients presenting with acute cardiogenic shock pathologies. Patients with HF-CS therefore have different needs regarding MCS. For example, a patient with chronic heart failure is much more likely to respond to an IABP, whereas a similar patient presenting with acute cardiogenic shock may require a more robust form of MCS. The study of temporary MCS in decompensated heart failure has been even more challenging than AMICS due of the diversity of etiologies of shock and the etiology of decompensation. The current use of specific devices is thus often dependent on the operator and institutions experience and expertise rather than clinical trials.
The most commonly used device for refractory acute decompensated HF-CS is an IABP. Despite several trials failing to demonstrate survival benefit in AMICS, there are data to suggest the effectiveness of IABP in HF-CS.29–31 Several retrospective studies have demonstrated use of an IABP resulting in acute stabilization and improvement in hemodynamic parameters in this subset.31–33 Malick et al. compared 73 patients with AMI-CS and 132 patients with HF-CS treated with IABP. The investigators found patients with HF-CS had cardiac output augmentation that was almost fivefold higher when compared to patients AMI-CS. The mean cardiac output augmentation was slightly >0.5 l/min in the HF-CS cohort, which may be enough improvement to stabilize such patients as they have a low cardiac output at baseline.34 Though not definitive, the study demonstrates well how the response to IABP is very dependent on the etiology of CS. There are plans for a well-powered trial evaluating the use of IABP in HF-CS.35
An Impella device is able to provide more cardiac output compared to IABP and has been increasingly used in HF-CS patients who decompensate despite optimal medical therapy.36–38 An Impella has the advantage of being able to fully unload the left ventricle and provide nearly complete ventricular support to most patients when using the larger 5.0 or 5.5 models.39,40 However, there are no trials to definitively prove mortality benefit.
Patients with refractory cardiogenic shock with concomitant respiratory failure may require full cardiopulmonary support with VA-ECMO and evidence for its utility in the treatment of cardiogenic shock is also increasing.41,42 A recent multicenter, retrospective cohort study of patients with mixed septic and cardiogenic shock demonstrated improvements in 90-day survival with VA-ECMO when compared to controls treated with conventional medical treatments alone; this was despite the fact that the treatment arm had greater degrees of organ and myocardial dysfunction compared with controls.43 Registry data from the Extracorporeal Life Support Organization in 2018 demonstrated a survival to discharge benefit for patients in refractory cardiogenic shock supported with ECMO.44 The magnitude of success for which depended on the etiology of the shock, with myocarditis patients faring the best and post-surgical patients doing the worst.45 This is an example of how the etiology of cardiogenic shock plays a key role in device selection and hemodynamic improvement with MCS. The optimal candidate for ECMO in cardiogenic shock remains an active area of investigation and there are multiple scoring systems being used to predict the outcome of ECMO in an individual patient with cardiac failure.45
Mechanical Circulatory Support for Right Ventricular Failure
Predominant RV failure constitutes up to 5% of patients presenting with AMICS, while concomitant RV failure in the setting of predominant LV failure is more common at 40%.46,47 RV failure can be identified by imaging as well as by hemodynamic parameters such as a central venous pressure (CVP) >12 mmHg, a CVP/pulmonary capillary wedge pressure ratio >0.63, a pulmonary artery pulsatility index <1.5, and a RV stroke work index <300 mmHg*ml/m2.
In AMICS, RV failure can be caused by right coronary artery occlusion. RV ischemia leads to decreased systolic function and decreasing trans-pulmonary flow into the LV. Decreasing preload results in decreasing cardiac output and hemodynamic compromise. RV failure should therefore first be treated with fluids, then consideration of inotropes. In patients with LV failure, increased LV pressures and pulmonary venous pressures leads to increased RV afterload and decreased RV function. The bulk of studies evaluating the use of RV MCS devices have therefore occurred in patients with RV failure in the setting of LV failure, such as in patients post-LVAD or those with chronic heart failure.
Gramega et al. conducted a small retrospective study that demonstrated improvements in systolic blood pressure, CVP and lactate with the use of an Impella RP in AMICS with RV failure.48 Cheung et al. studied 18 patients, 39% of whom had AMI, and found that the Impella RP led to improvements in hemodynamic measures and reported a 30-day survival rate of 72% and a 1-year survival rate of 50%.49 Anderson et al. conducted a prospective study in 2018 including 60 patients. Patients were divided into two cohorts based on the etiology of their RV failure. One cohort was post-LVAD implantation and the other post-surgery or AMICS. Similar to other studies hemodynamics improved, including cardiac index and CVP along with decreasing use of inotropes.50 Kapur et al. performed a retrospective study evaluating 46 patients with predominant RV failure of various etiologies and sought to evaluate the hemodynamic effects and clinical outcomes of using either a percutaneously or surgically cannulated TandemHeart-RV support device. Hemodynamic parameters including mean arterial pressure, cardiac index and CVP significantly improved the use of RV-MCS.51 VA-ECMO is a powerful RV-MCS as it can bypass the venous system entirely. Particularly in the setting of concomitant left-sided failure, ECMO may be the preferred MCS modality as it provides biventricular support. Unfortunately, data on its use specifically for RV failure are limited.
Conclusion
MCS devices are increasingly used for the treatment of cardiogenic shock. Clinicians use these devices to improve hemodynamics in an effort to support coronary and systemic perfusion. These devices work through various mechanisms, resulting in different physiological responses. There is an increasing effort to enroll and study patients requiring MCS into studies to help refine patient and device selection. Current practice using shock protocols and multidisciplinary teams takes into account operator and institutional experience and expertise to allow for a safe delivery and use of such devices. When choosing an MCS device for a patient, clinicians must take into account the etiology of shock (acute versus acute on chronic pathologies), the stage of shock (including the extent of end-organ failure) and the patient’s age and beliefs along with the goals of care. With continued technological advancements these devices will continue to become smaller and more powerful, thus clinicians should be familiar with the risk and benefits of such devices.