Shock is the physiologic state of reduced tissue perfusion resulting in anaerobic metabolism, cellular injury, and ultimately death. Tissue perfusion is maintained by adequate cardiac output (CO) and sufficient systemic vascular resistance. Cardiogenic shock (CS) is a decrease in CO leading to a state of systemic hypoperfusion and accounts for 100,000 hospitalizations annually in the US, with a reported in-hospital mortality of 27.1–41%.1–3
Historically, landmark trials have used various definitions of CS. However, it is generally accepted that refractory cardiogenic shock (rCS) is defined as systolic blood pressure ≤90 mmHg for longer than 30 minutes or when vasopressors are required to achieve a systolic blood pressure ≥90 mmHg, severely reduced cardiac index (≤1.8 l/min/m2 or ≤2.2 l/min/m2), elevated biventricular filling pressures (central venous pressure ≥10mmHg; pulmonary capillary wedge pressure ≥15 mmHg) and evidence of end-organ dysfunction related to hypoperfusion such as an arterial lactic acid >2.0 mmol/l and/or a low mixed venous oxygen saturation despite maximal pharmacological interventions such as inotropes and the above-mentioned vasopressors.4–10 Recently, the Society for Cardiac Angiography and Interventions (SCAI) published an expert consensus statement to emphasize that CS is a continuum rather than being simply present or absent in an effort to facilitate early recognition of progressive shock.11
Cardiac arrest (CA) shares a similar, albeit more imminent, final common pathway with rCS. During CA, all CO ceases, leading to low end-organ perfusion even with optimal cardiopulmonary resuscitation (CPR), and ultimately death if the return of spontaneous circulation is not achieved. Out-of-hospital cardiac arrest (OHCA) affects approximately 378,000 patients/year in the US with a survival rate of 10.6%; and 8.5% of the total survive with good neurologic status.12 In-hospital cardiac arrest has a slightly better mortality outcome (26.7% of whom 80.3% have good neurologic status) but, overall, prognosis is still quite grim.12
The high mortality associated with both rCS and CA coupled with the failure of advances in care to improve outcomes in the past decade have made veno-arterial extracorporeal membrane oxygenation (VA ECMO) an attractive rescue strategy to provide immediate perfusion and pulmonary support while investigating and correcting the underlying pathology.1–3,12–17 Consequently, the use of VA ECMO in the management of rCS and refractory CA has increased.1–3,17–19
While many patients with CS or CA will benefit from VA ECMO, the overall outcomes for patients placed on VA ECMO remain less than ideal. To this end, it is incumbent upon the cardiology, critical care and cardiothoracic surgery communities to identify patients who may benefit in the form of neurologically intact survival, improve delivery of VA ECMO and increase the understanding of the best practices for managing VA ECMO in an effort to minimize complications and optimize recovery in a timely and cost-effective manner.
In this review, we focus on patient selection, principles of VA ECMO, contraindications, complications, and management including care after a cardiac arrest.
Patient Selection for VA ECMO in Cardiogenic Shock and Cardiac Arrest
Cardiogenic Shock
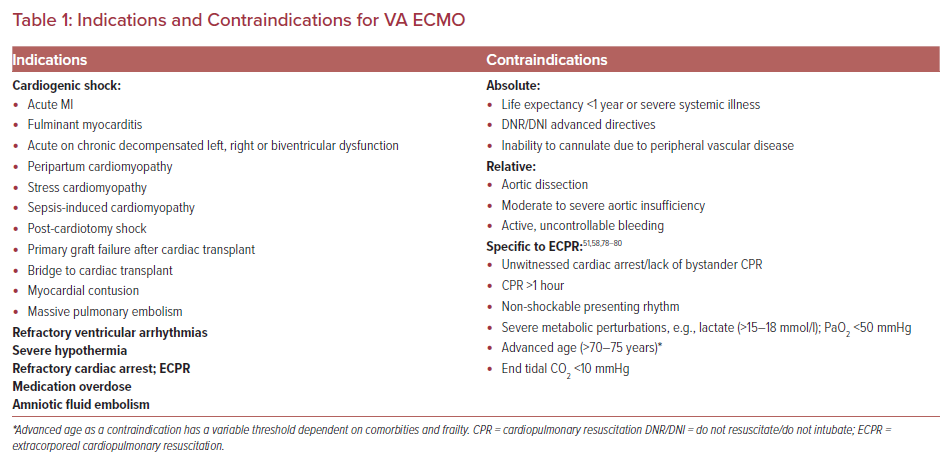
The etiologies of CS are broad; some are listed in Table 1. Recognizing the etiology and establishing the SCAI stage of CS rapidly is critical because ongoing use of high-dose pharmacologic agents such as inotropes and vasopressors may prove inadequate or cause unintended side effects including arrhythmias and increased myocardial oxygen consumption.20 These may hinder myocardial recovery or even become life threatening if mechanical circulatory support (MCS) devices are not offered.
Providers have various considerations when deciding if MCS is appropriate. Although a complete understanding of a particular patient’s prognosis is lacking, timely decisions regarding escalation to MSC are critical before irreversible damage from poor perfusion to end organs leads to catastrophic, irrecoverable injury; ideally, it should be initiated within 60 minutes of recognition of rCS.21 In these circumstances, MCS may be viewed as a bridge to recovery, to a decision or to a more definitive therapy such as permanent left ventricular assist device (LVAD) or heart transplant if the patient is a candidate.
Further complicating the decision to offer mechanical support in the form of VA ECMO is the lack of randomized controlled trials supporting improved mortality; however, three randomized controlled trials (RCTs) – EURO-SHOCK , ANCHOR (NCT04184635) and ECLS-SHOCK (NCT03637205) – are expected to provide clarity regarding mortality benefits associated with MCS in the population of patients experiencing CS at or around the time of an acute coronary syndrome event.22
Once the need for MCS has been identified, consideration should be given to the etiology of CS as there is significant heterogeneity in survival across groups.23,24 For example, patients with fulminant myocarditis and primary graft failure after heart transplantation have a better prognosis likely owing to the higher chances of myocardial recovery.23–26
The Survival After Veno-arterial-ECMO (SAVE) score and the Prediction of Cardiogenic Shock Outcome for acute MI Patients Salvaged by VA ECMO (ENCOURAGE) score have been developed based on pre-ECMO risk factors associated with poor outcomes in an effort to help facilitate the selection process for VA ECMO cannulation in rCS patients.27,28 These scores use variables that have been associated with higher mortality in patients placed on VA ECMO, including advanced age (increased risk with increased age), female sex, higher weight, impaired renal and/or liver function, previous cardiac arrest, central nervous system dysfunction, duration of intubation, peak inspiratory pressure, as well as markers of severity of cardiac dysfunction, such as lower pulse pressure prior to ECMO (<20 mmHg), elevated lactate (increasing risk with increasing levels above 2 mmol/l), reduced prothrombin activity (<50%) and elevated diastolic blood pressure prior to initiation of ECMO (>40 mmHg).27–30
Cardiac Arrest
CA is divided into shockable, ventricular tachycardia (VT) and ventricular fibrillation (VF), and non-shockable, pulseless electrical activity (PEA) and asystole rhythms.
PEA is cardiac electrical activity that does not result in meaningful CO and is often caused by obstruction of blood flow leading to poor cardiac filling (massive pulmonary embolism, cardiac tamponade or tension pneumothorax) or profound loss of systemic vascular resistance (SVR) owing to metabolic perturbations. Low SVR ultimately leads to a precipitous fall in preload, resulting in low or no CO.
Patients with VT/VF have a significantly lower mortality than those presenting in PEA arrest, in part, because of the reversible nature of the underlying pathophysiology where VT/VF is frequently seen in the setting of acute MI.31 Within minutes of myocardial ischemia, there are changes in membrane potential, calcium transport, and intracellular concentrations of potassium, which lead to a heterogeneous refractory period and an environment ripe for micro reentry circuits. In scarred myocardium or dilated cardiomyopathy, macro re-entry circuits exist that lead to VT/VF. Without intervention, VT/VF will inevitably progress to asystole, so it can be inferred that if a presenting rhythm is VT/VF rather than asystole, there is a higher likelihood the patient has had a shorter duration CA, which is associated with a better prognosis owing, in part, to a shorter duration of hypoperfusion.
VT/VF occurs in 15–30% of all OHCA patients in the US with some emergency medical services reporting higher and some lower numbers.1 However, 60–80% of all CA survivors with neurologically favorable function come from this group.13 Despite the more favorable prognosis, only 35–50% of treated VT/VF patients overall survive to discharge.31 Presumably, some portion of this group develops refractory VT/VF (rVT/VF) and patients are declared dead before admission. rVT/VF is typically defined as VT/VF that persists despite at least three defibrillation attempts during standard resuscitative efforts. Taken together, this suggests VT/VF patients have the highest potential for recovery, presenting a group in which further attempts at immediate and advanced cardiorespiratory support may be of significant benefit. Of patients with rVT/VF, 70–85% have underlying acute or chronic coronary artery disease.12,13,32–42 This supports the notion there is a potential for curative interventions if end-organ damage owing to the CA can be limited by extracorporeal cardiopulmonary resuscitation (ECPR).33,34,41
ECPR survival has historically been low, in a range of 8–38%.18,43–48 The 2020 Extracorporeal Life Support Organization (ELSO) registry reported 29% survival to discharge among ECPR patients while the total population of VA ECMO patients survival to discharge rate was 45%.17 These comparatively dismal statistics in the setting of a potentially reversible etiology has stimulated continued interest in improving the use of ECPR as a rescue therapy.
The International Liaison Committee on Resuscitation advanced life support task force commissioned a systematic review in 2018 that concluded ECPR could be considered for select patients when conventional CPR was failing (weak recommendation, low certainty of evidence).49 Similarly, the 2020 American Heart Association resuscitation guidelines offer a 2b recommendation for ECPR, citing 15 observational studies, most of which reported improved neurologically intact survival, but there were no RCTs to support the use of ECPR.50,51 Confounding these data were highly variable inclusion criteria, ECMO settings, study design, and possible selection bias.51 However, the ARREST trial, a highly anticipated prospective RCT demonstrated improvement in neurologically favorable outcome with ECPR (42.9%) compared with standard advanced cardiac life support (6%) in 30 patients. While this was a single-center, open-label trial, the promising results warrant further investigation.52
At this time, there are no clear, society-endorsed guidelines regarding when and in whom VA ECMO should be used. Given this, it is widely accepted that institutional experience will impact outcomes for this highly technical procedure that is time sensitive and requires specialized management.53 The best and most consistent outcomes in ECPR patients seem to be paired with a highly structured, community-wide approach focused on early, effective CPR followed by short-duration to VA-ECMO insertion and minimization of VA ECMO-induced complications that ultimately affect survival.54 These efforts include: programs to increase bystander CPR facilitated by 911 dispatchers; early patient identification by highly trained paramedic teams; the use of mechanical CPR devices during transport to ensure high-quality uninterrupted CPR; clear algorithms for paramedics regarding transport of patients to facilities capable of initiating VA ECMO; a specialized team available for emergent (team available within 30 minutes/on arrival to hospital, 24/7) ultrasound-guided, fluoroscopically confirmed cannulation; and, finally, a centralized intensive care unit (ICU) for post-cannulation care with technically trained nursing staff and critical care cardiologists.32,33,55,56 In an effort to further reduce the duration of low-flow state associated with CPR, some groups have trialed cannulation in a variety of settings including the emergency room and in the field. 56–58
In this emerging field, for now, it is common to rely on expert opinion and institutional experience in the decision to implement ECMO as a rescue strategy for either CA or CS. While both SAVE and ENCOURAGE scores exclude ECPR patients and the SAVE score specifically does not correlate with mortality in the ECPR population, there is some evidence that similar risk factors, among others, may influence mortality.59 In ECPR, younger age, witnessed arrests, rhythm other than asystole and recovery of mean arterial pressure are predictive of good outcomes.47,60 The ECPR score makes an effort at using these risk factors in a risk prediction model for surviving to discharge in CA patients placed on ECPR.61
In general, patient selection for VA ECMO in rCS continues to evolve but generally revolves around myocardial recovery potential or an exit strategy to some longer-term support options and selection for younger patients with few comorbid conditions. Patient selection in CA remains more opaque and decision making is more complex because interventions are needed immediately. Specific algorithms used by mature ECPR programs, similar to those presented in the ARREST trial, will begin to shape our understanding as to which patients may benefit from this rescue strategy.52
Basic Principles and VA ECMO Circuit Set-Up
VA ECMO provides full cardiopulmonary support to patients in CA or CS with or without concomitant respiratory failure. The VA ECMO circuit consists of an inflow cannula that pulls deoxygenated blood from the venous system via a centrifugal pump. Blood is passed through a hollow-fiber membrane oxygenator or blood-gas exchange unit for removal of carbon dioxide and oxygenation then it is returned, via an arterial (outflow) cannula to the systemic circulation.62 The inner surface of the circuit tubing is typically coated with heparin to minimize complement activation, platelet adhesion and inflammation.63 Notably, this tubing is safe in patients with heparin-induced thrombocytopenia due to covalent binding of the heparin to the artificial surfaces.64
VA ECMO can be implemented in two forms, with the nomenclature reflecting cannulation site. Central VA ECMO can be placed by midline sternotomy or thoracotomy with cannulation of the superior vena cava, inferior vena cava, or, most commonly, the right atrium for venous access, and the aorta, subclavian/innominate or pulmonary artery for arterial return. Peripheral VA ECMO uses large-bore cannulas placed percutaneously or via Dacron grafts placed by surgical cutdown in one of several configurations. Venous access is gained through the femoral vein most commonly during CA due to the ease of cannula insertion, but, alternatively, the right internal jugular vein can be utilized.
Arterial access is most often gained in the femoral artery, although severe peripheral vascular disease can limit this option and the subclavian artery can also be accessed. It is notable that hyperperfusion of the upper extremity in the latter scenario can lead to complications such as compartment syndrome, but it does have the added benefit of potential for ambulation if paired with right internal jugular venous access.65
In femoral cannulation, the distal tip for the arterial cannula typically lies in the descending aorta or common iliac artery and the distal tip of the venous cannula lies somewhere between the superior vena cava, right atrium and inferior vena cava depending on the approach and patient size.53,66 The venous cannulas are typically multistaged, which means there are multiple perforations at various points to allow flow along the cannula. Arterial cannulas are 15 cm or 23 cm long and range in size from 15 Fr to 21 Fr, while venous return cannula are 55 cm long and range in size from 21 Fr to 29 Fr, with the venous cannula diameter typically the flow-limiting component in the circuit.53
The amount of circulatory support provided by VA ECMO is determined by the flow rate through the circuit, which is set by adjusting the revolutions per minute on the pump. The initial goal is typically 50–70 ml/kg/min (about 3–6 l/min) and a mean arterial pressure of >60 mmHg.53 The extent of ventilation or carbon dioxide removal and oxygenation is adjusted by modifying the sweep or countercurrent gas flow and the fraction of inspired oxygen (FiO2) through the oxygenator, respectively.53,62
Due to efficiency of the oxygenator, full respiratory support can typically be provided to allow for full pulmonary rest, minimizing barotrauma so long as an adequate portion of the total CO is passing through the circuit.67 Likewise, the efficiency of the oxygenator increases the risk of hyperoxia, which is associated with worse outcomes particularly in post CA patients.68–70 Finally, most circuits contain a heater/cooling system to return blood at a set temperature. This is particularly useful with targeted temperature management in post-CA patients.
Contraindications
Absolute contraindications for VA ECMO are largely based on expert opinion and somewhat fluid (Table 2). They typically include a life expectancy of less than 1 year even with successful cardiac recovery, disseminated malignancy, previous end-stage organ failure, severe irreversible brain injury, and/or patient goals that limit aggressive measures.
Severe peripheral arterial disease can be an absolute contraindication for peripheral cannulation if access is not obtainable. Moderate to severe aortic regurgitation is at least a relative contraindication owing to the retrograde flow of VA ECMO causing severe left ventricular dilatation and subsequent pulmonary edema.
Some relative contraindications require a multidisciplinary team discussion before proceeding. For example, some patients in whom there is no clear exit strategy in the case of failure of myocardial recovery may benefit from evaluation and consideration for a trial of cannulation as a bridge to recovery. The presence of an aortic dissection is another relative contraindication due to risks of additional fenestrations and false lumen cannulation.71–74
Other relative contraindications include advanced age (typically >70–75 years), bleeding diathesis and prior aortic or mitral valve prosthesis due to decreased flow increasing risk for valve thrombus.75–77 The specific contraindications of ECPR are not well defined and exclusion criteria vary across studies. Table 1 outlines the commonly used exclusion criteria.52,56,58,78–80
Complications
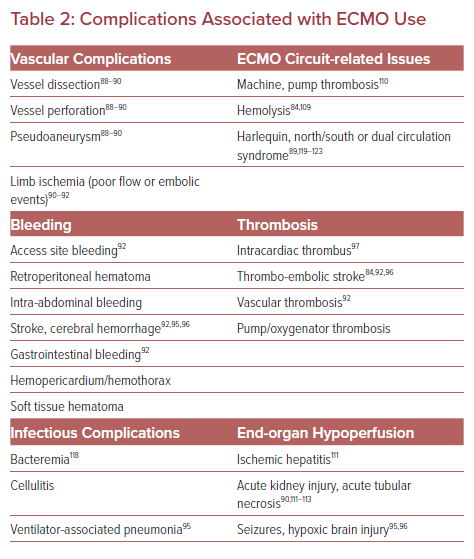
The literature surrounding complications associated with VA ECMO are highly heterogeneous with no standardized definitions. Most data are from observational studies or case reports and lack granularity. A broad range of complications are reported (Table 2), some associated with high morbidity and mortality, that must be prevented if possible, recognized early, and treated promptly when necessary.
Vascular Injuries and Leg Ischemia in Peripheral VA ECMO
Vascular complications are reported at rates of 20–30% and often potentiated due to systemic anticoagulation (AC) for the ECMO circuit (Table 2).18,19,81,82 Distal limb ischemia with peripheral cannulation is relatively common, with a reported prevalence of 17–40%.18,19,25 The risk is higher if the target vessel's diameter is not at least 1–2 mm larger than the cannula diameter.62
Additionally, leg ischemia has been associated with female sex, younger patients (30–40 years vs 50–60 years) because of to smaller vessel size and fewer collateral vessels, severe peripheral arterial disease and a cannula size over 20 Fr. 83,84 This issue has largely been addressed by the routine insertion of a distal perfusion catheter (DPC). Typically, a 5–8 Fr cannula is inserted into the superficial femoral artery or posterior tibial artery, which redirects a small portion of the arterial return flow from the ECMO circuit to the distal circulation of the cannulated limb.
Lamb et al. described leg ischemia in 33% of patients who did not receive DPC and in none of those with a catheter; the absence of leg ischemia was associated with increased survival.83 Limb perfusion should be monitored using physical examination or near-infrared spectroscopy (NIRS) placed on the bilateral calves in addition to routine Doppler evaluation of the distal extremity regardless of the presence of a DPC. Highly trained and experienced teams have lower complication rates, and percutaneous access VA ECMO initiation has lower rates of complications than surgical or hybrid approaches.52,85
Bleeding and Thrombosis
There is a delicate balance between bleeding and thrombosis risk in patients on VA ECMO, with both often occurring simultaneously. Bleeding complications occur in 18–56% of patients.23,24,28,30,44,45,86 However, the VA ECMO circuit is itself considered prothrombotic due to blood exposure to synthetic surfaces, endothelial injury during vascular access, shear stress and platelet activation, and consumptive coagulopathies leading to hemostatic imbalances.87,88 Bleeding complications vary in severity (Table 2). Perhaps the most consequential – intracranial hemorrhage – occurs in 2–3% of patients, and has a mortality rate of near 90%.53,86,89
Similarly, a hypercoagulable state can lead to thromboembolic events, including stroke (4–7%), limb ischemia and intracardiac thrombus, or aortic root thrombus, particularly if antegrade CO is low.77,86,89,90 Rarely, machine failure can cause thrombosis/embolization of the oxygenator or the pump. For these reasons, it is standard to use systemic anticoagulation (AC) while patients are supported with VA ECMO.77
Although debated, the use of hollow-fiber polymethylpentene oxygenators, heparin-coated tubing, and newer centrifugal pumps with limited heat generation and thrombogenicity are thought to have reduced the overall hypercoagulability of the circuit.91–94
Given these complexities, guidelines for optimal AC rely on expert opinion and there is considerable variation between institutional practices. These range from no AC to holding AC for up to 3 days in the setting of bleeding while flow remains over 3 l/min, to use of direct thrombin inhibitors such as argatroban or bivalirudin as the anticoagulant of choice, especially in the setting of heparin-induced thrombocytopenia.95–99
The ELSO 2014 AC guideline recommends that patients on VA ECMO should be targeted to an activated coagulation time (ACT) of 180–220 seconds with the use of unfractionated heparin.100 Given concerns over the poor association between ACT value and bleeding events, many institutions have moved to an anti-Xa assay strategy with a goal ACT of between 0.3 and 0.7 seconds despite little evidence in VA ECMO patients. Some institutions use partial thromboplastin time (aPTT) and anti-thrombin III assays to further refine their AC strategy with heparin.101,102
Daily evaluation for clot formation with visual inspection of the oxygenator as it is the most common site for thrombus formation should be carried out and measures of hemolysis such as lactate dehydrogenase, plasma-free hemoglobin, and bilirubin should be monitored.77,102,103 Excessive hemolysis can be seen after large transfusions and with hematoma absorption of hematoma but also related to excessive ECMO flow/pump speed, a too-small cannula, high negative venous pressures (usually associated with circuit ‘chatter’ or swinging of the circuit tubing that occurs when maximum blood flow rate has been exceeded due to venous collapse), pump thrombosis, or a clot in the oxygenator, which would suggest a patient may benefit from modification or exchange in the circuit.
Liver and Kidney Injury
Injury to the liver (hyperbilirubinemia; 12%) and kidney (12–56%, with need for hemodialysis in ~12–15%) are common in patients on VA ECMO.83,104–108 However, it is difficult to differentiate between injury related to the inciting CS, CA or other therapies such as drug toxicity, or hypotension from injury related directly to ECMO.
Masha et al. showed that, in 223 patients on VA ECMO, an increase in total bilirubin significantly correlated, in a linear fashion with mortality. In addition, no patient with a bilirubin level greater than 30 mg/dl survived, and a bilirubin level of approximately 11 mg/dl was the threshold for 90% mortality in univariate analysis, which suggests that bilirubin is an important marker of prognosis in patients on VA ECMO support and may be a sign of intolerance of the circuit.109
Alkaline phosphatase has also been reported as a predictive marker for mortality in VA ECMO.110
Infections
As with any indwelling catheter in place for a prolonged period of time, VA ECMO can be associated with cellulitis, bacteremia, and sepsis; this affects 3–18% of patients and is associated with mortality as high as 64%.81,111 In addition to line/circuit-associated infections, patients in this population are also at risk of pneumonia as with all those in ICU who require mechanical ventilation.
Harlequin, North/South, or Dual Circulation Syndrome
Harlequin syndrome is a well-described phenomenon unique to the femoral VA ECMO set-up. Cardiac contractility recovers in this scenario while alveolar gas exchange remains inadequate due to either insufficient ventilator settings or ongoing severe lung injury. Native CO increases and therefore poorly oxygenated, carbon dioxide-rich blood leaves the left ventricle (LV). Consequently, a mixing cloud develops in the proximal ascending aorta and moves distally as the native CO increases, pushing the reach of oxygenated blood provided by the ECMO circuit further distal in the aorta.
Signs of this are decreased oxygen delivery to the first branches of the ascending aorta, including the coronary arteries and the innominate artery leading to decreased oxygen delivery to the cerebral and right subclavian vessels. Accordingly, right-hand saturation and arterial blood gas monitoring and/or new or increasing discrepancy in upper extremity NIRS monitoring are critical for early identification.82,112–116 If unrecognized, this syndrome can lead to prolonged hypoxia of myocardial tissue and anoxic brain injury.
Ways to combat this phenomenon include modifying ventilator settings, increasing ECMO flow, and decreasing inotropic support, and, if these measures fail, conversion to veno-arterial-veno (VAV) configuration can be considered.111, 117–119 In the VAV setup, a portion of the oxygenated blood returning from the circuit is diverted to a second outflow cannula (flow controlled with a roller clamp) placed in a central vein with outflow at or near the right atrium. It provides pre-oxygenated blood that circulates through the pulmonary vasculature and, ultimately, is ejected from the left ventricle. Therefore, oxygen-rich blood flow to the proximal aortic branches will be restored.
Other Management Issues
Left Ventricular Unloading
The physiologic changes noted in CS in the setting of left heart failure are primarily due to a decline in LV contractility. This leads to reduced stroke volume (SV), high LV end diastolic pressure (LVEDP), high pulmonary capillary wedge pressure and a neurohormonal-reflex mediated increase in systemic vascular resistance.120–122 By diverting venous blood flow into an external circuit, VA ECMO decreases systemic venous congestion and right ventricular preload.120,123,124
However, the hemodynamic effect on the LV remains debated. Observational clinical and translational studies using computer modeling suggest higher LV stroke work and LVEDP after VA ECMO is started owing to an increase in LV afterload caused by retrograde return of blood into the arterial circulation.125–127 It has been hypothesized that increased afterload increases LVEDP and decreases SV and CO. Consequently, this increases stroke work, resulting in a decrease in coronary perfusion pressure and, in so doing, worsening myocardial ischemia and/or adversely affecting myocardial recovery.128–131 Simultaneously, increased afterload may reduce the opening of the aortic valve with each cardiac cycle because the LV is unable to generate pressures higher than the aortic pressure, leading to stasis of blood in the LV and thrombi formation.132,133 Conversely, recent clinical experience, including evidence from the ARREST trial and others, suggests VA ECMO support alone provides a favorable environment for myocardial recovery.52,134
Given the concerns that VA ECMO may impair myocardial recovery, there has been increased use of various LV unloading strategies. These include the infusion of inotropes or vasodilators for afterload reduction, the use of percutaneous mechanical assist devices such as an intra-aortic balloon pump (IABP) or Impella, transseptal left atrial cannulation devices, and surgical LV venting.120,127,135–143 Strategy selection often depends on patient comorbidities, complications, resource availability, and institutional preference.144
The efficacy of the most broadly used strategies, the IABP or Impella in combination with ECMO, remains largely understudied on a prospective basis.145 Meta-analyses evaluating concomitant IABP use with VA ECMO versus VA ECMO alone have not identified substantial improvement in mortality among patients with CS or CA.146–148 However, in subset analyses of patients with CS secondary to acute MI, the addition of IABP to VA-ECMO was associated with lower mortality.
The addition of an Impella to VA ECMO for LV unloading has been predominantly evaluated in animal, observational, and retrospective studies to date.149–150 Schrage et al. recently assessed the impact of VA-ECMO plus Impella versus VA-ECMO alone in CS in a 1:1 propensity-score-matched cohort.127 The VA ECMO plus Impella group was associated with a lower 30-day mortality but had a higher rate of complications including severe bleeding, access site-related ischemia and renal replacement therapy.
A meta-analysis of 17 observational trials including patients with CS found survival benefit with an LV unloading device (IABP, Impella or transseptal LA cannula) compared to ECMO alone and found no significant difference in bleeding, organ failure, stroke, and limb ischemia.148
To date, observational clinical data favor the use of mechanical LV unloading devices in addition to ECMO with appropriate patient selection; however, clinical investigation, hemodynamic data, and physiologic changes related to each method are urgently needed to optimize future care and costs associated with VA ECMO.
Weaning and Decannulation
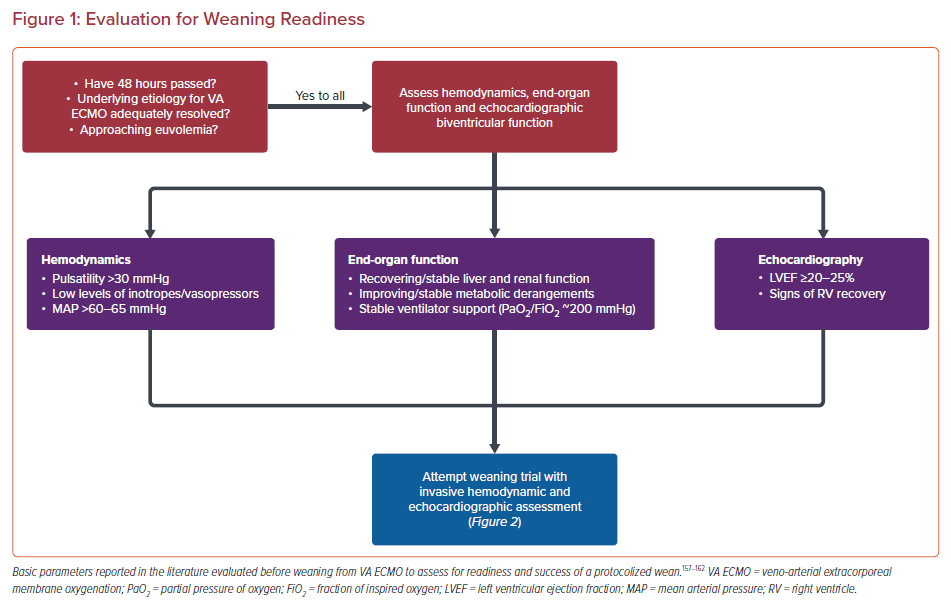
VA ECMO-weaning protocols, if available, vary highly between institutions, reflecting the limited literature on the topic. Only a few articles include data from large cohorts and none have a prospective approach to compare methods.151 The minimum requirement for readiness for weaning are subject to debate. In general, a weaning trial can be pursued after some degree of myocardial recovery is seen, usually after 48 hours of cannulation,improvement in liver function, and only minimal hemodynamic/respiratory support is required.53,152–156 However, what defines minimal support is debated.
Pulse pressure waves are typically small or flat when non-functioning or minimally functioning myocardium is paired with relatively higher VA ECMO flows. Predictably, pulse pressure waves increase upon myocardial recovery. While no exact pulse pressure threshold has been established, higher pulse pressure is considered a clinical marker for readiness for a weaning trial and has been associated with weaning success.152,157,158
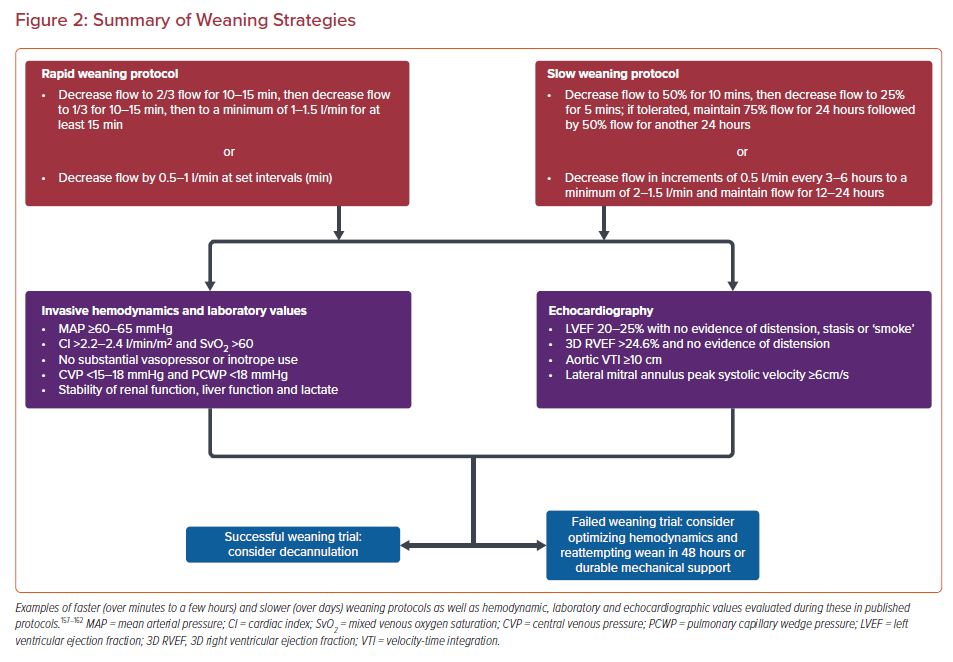
In addition to identifying predictors of success, a multidisciplinary discussion between the cardiology team, heart failure specialists, intensivists, and cardiothoracic surgeons, as well as the patient and/or their family should be considered in case of weaning or decannulation failure. Weaning algorithms that predict successful decannulation include various combinations of echocardiographic, invasive hemodynamic, and biomarker data, collected as the VA ECMO flow is slowly decreased to 1–1.5 l/min (Figures 1 and 2).77,159
Importantly, it is generally accepted that the risk for thrombus formation increases at ECMO flows below 2 l/min, and adequate AC is strongly encouraged when proceeding with the turn-down study.53 LV echocardiographic data that predicts successful weaning include higher aortic velocity-time integrals (>10 cm), LV ejection fraction (20–25%), and lateral mitral annulus peak systolic velocity (>6 cm/s) while mitral E/tissue Doppler Ea’ suggesting higher filling volumes predict worse outcomes.152
Evaluation of right ventricular parameters predictive of weaning success are less robust. A small cohort study showed that a 3D right ventricle ejection fraction of >24.6% was associated with higher weaning success and lower 30-day mortality.160 Although it is not approved in the US, a handful of studies have looked at pretreatment with levosimendan and found it may increase the chances of weaning success.161,162
ICU Considerations
Details of ICU post-arrest management are beyond the scope of this review, but the complexities of care in this setting, including nuances of targeted temperature management, post-arrest hemodynamic goals, neuroprotective strategies such as permissive hypercapnia, and oxygenation strategies, among others, underline the importance of having a multidisciplinary team caring for VA ECMO patients.163–191 Specialists including heart failure, critical care, cardiothoracic surgery and/or vascular surgery, nephrology, palliative care and neurology physicians along with perfusionists, respiratory therapists, pharmacists, nutritionists, and specially trained nurses for a framework to care for some of the most medically complex patients in the hospital.75,192–194
While some patients will recover cardiac function allowing liberation from ECMO, others will not. It is prudent for the team caring for a patient to prioritize early identification and planning for patients who need long-term mechanical support or transplant evaluation. Commonly, the assessment for appropriateness for advanced options takes time and, if delayed, complications related to VA ECMO can become barriers to eligibility.
Conclusion
In summary, VA ECMO offers an appealing salvage therapy to patients who likely would not otherwise have any chance of survival. Our understanding of how to more effectively deliver VA ECMO combined with advances in technology have manifested as increased use of ECMO and have been bolstered by early signs in the literature that we may be able to improve outcomes. Nonetheless, knowledge gaps persist, mortality remains suboptimal, and widespread reproducibility is difficult. Expert opinion and institutional preferences largely dominate care. More rigorous prospective RCTs similar to the ARREST trial are desperately needed to standardize care in the form of guidelines to maximize survival for patients.