Tricuspid regurgitation (TR) is a challenging disease with adverse clinical outcomes if left untreated.1,2 The prevalence and severity of TR are directly proportional to age, and the 1-year survival rate for patients with severe TR is approximately 60%.3,4 TR is classified as primary or degenerative and secondary or functional, with approximately 90% of TR being secondary.5 Primary TR is caused by an abnormality in the structure of the tricuspid valve (TV), and is seen with infective endocarditis; congenital heart conditions, such as Ebstein disease; pacemaker lead-induced leaflet perforation; carcinoid tumor; and rheumatic heart disease. Secondary TR is usually caused by leaflet malcoaptation due to dilatation of the annulus or leaflet tethering in the setting of right ventricular (RV) disease, left-sided valvular disease, myocardial dysfunction, or pulmonary hypertension (pHTN).6 Severe TR results in symptoms of right heart failure (RHF), venous congestion, and low cardiac output.7 Once severe RV dysfunction ensues, valvular intervention may not help.8 However, the guidelines are not precise about defining RV dysfunction for contraindication of intervention.9
Due to the highly compliant nature of RV, even severe TR remains clinically unnoticed until the late stages of the disease. The medical management of TR includes the control of symptoms related to fluid overload with diuretic therapy and the treatment of RHF when it occurs. Therapies to treat the primary etiologies of heart failure, such as vasodilators for pHTN, guideline-directed therapy for left ventricular dysfunction, or rhythm control agents, are indicated.10 Although medical management helps with symptom control, it does not reverse the pathology of TR. Definite treatment of TR depends on the etiology of TR, and surgical TV repair (TVr) or TV replacement (TVR) should be considered when medical management fails. According to the 2020 American College of Cardiology/American Heart Association guidelines, TV surgery is recommended for patients with severe TR undergoing left-sided valve surgery (class 1) or for isolated severe primary TR in patients with RHF to reduce symptoms and recurrent hospitalizations (class 2a).10 Surgical TVr or TVR is indicated for severe, symptomatic TR, or asymptomatic severe TR with progressive RV dysfunction. TVr is also indicated with >40 mm dilatation of the tricuspid annulus on transthoracic echocardiogram (TTE) or >70 mm (>21 mm/m2) on direct intraoperative measurement.11 Surgical TVR or TVR for isolated TR carries a high overall mortality of 8.6%.12 Most patients with severe TR are at high perioperative risk or inoperable. Transcatheter TV repair (TTVr) or transcatheter TV replacement (TTVR) are potential options for patients who are not surgical candidates. TTVr with MitraClip, Tricuspid clip, or Pascal device is an alternate treatment option for patients with severe TR who are at high or prohibitive surgical risk.13–16 However, TTVr may not be feasible in certain situations, such as large coaptation gaps >8 mm; calcified or severely tethered leaflets, where leaflet grasping is difficult; certain anatomies of the TV, such as Ebstein anomaly, causing tricuspid stenosis; or the presence of pacemaker leads in the TV.17 In such situations, TTVR is better suited to treat the TR. There are two types of TTVR, orthotopic and heterotopic. Orthotopic TTVR involves positioning the prosthetic valve in the tricuspid annulus, while heterotopic TTVR involves positioning the transcatheter valve in the superior vena cava or inferior vena cava (IVC).18
Small studies have shown the feasibility and efficacy of TTVR in patients with severe symptomatic TR who are at high surgical risk. A small single-center study by Hahn et al. showed promising results of TTVR, with a significant reduction in TR severity and New York Heart Association class, and improvement in RV function with the NaviGate TV system.19 Another recent compassionate study of 25 patients who underwent transfemoral EVOQUE TTVR demonstrated a 30-day mortality of 0%.20 With emerging technology and expertise, TTVR may become an attractive option for patients with prohibitive surgical risk or ineligibility to TTVr.21 This review highlights patient selection for TTVR, various types of TTVR devices, technical considerations for the procedure, and procedural planning for orthotopic TTVR (prosthetic valve deployed at the tricuspid annulus).
Patient Selection
Several important factors must be considered while selecting patients for TTVR. In general, patients with moderate-to-severe TR at high or inoperable risk for surgery and valve anatomies unsuitable for TTVr may be evaluated for TTVR candidacy. Several conditions that have lower chances of optimal technical success with TTVr, such as primary TR with fibrotic or calcified leaflets, especially at anchor site, severe prolapse, or severe annular or RV dilatation and extreme leaflet tethering with a large coaptation gap (>8 mm), may be better suited for TTVR.22 TTVr is also less successful in TR with eccentric regurgitant jets.23 In contrast, TTVR success is not dependent on leaflet anatomy.
Another important consideration in patient selection is RV and pulmonary hemodynamics. This applies to both surgical and transcatheter replacement. Restoring valve competency after TV replacement in patients with severe RV dysfunction may lead to RV–pulmonary arterial coupling mismatch.24 It may cause hemodynamic instability due to the worsening of RV dysfunction, often requiring circulatory support. For this reason, patients with severe RV dysfunction were excluded from the multicenter TRISCEND trial using the EVOQUE valve. One-quarter of patients receiving TTVR showed worsening RV function postoperatively; however, the rate came down to 4.5% at 30-day follow-up.25 The TRISCEND II trial is in the recruitment phase (NCT04482062). Patients with severe pHTN are at accelerated risk of afterload mismatch, although there are no clear data on thresholds.20,26,27 In surgical replacement, severe pHTN has been associated with high mortality.28 Such patients should receive aggressive medical management and be reassessed for TTVR candidacy. Patients with pulmonary artery systolic pressures >55 mmHg were excluded from some TTVR trials (NCT05194423).
Since blood flows at lower velocities across right-sided valves, patients who undergo TTVR need long-term anticoagulation to prevent prosthetic valve thrombosis.29 Thus, TTVr is probably better suited for patients with a high risk of significant bleeding if anatomy allows it. Patients with permanent pacemaker wires across the TV pose a unique challenge to both surgical and transcatheter TV interventions. Compared with TTVr, these patients are better suited for TTVR, which can be performed safely without lead extraction.30 However, a recent study presented at the Heart Rhythm Society 2023 meeting suggests jailing pacemaker leads may be harmful in patients who are pacer-dependent or have an ICD.31 Finally, a careful assessment of comorbid conditions, functional status, and frailty are paramount to the success and outcomes of any procedure. Patients with a life expectancy <1 year would be deemed poor candidates for TTVR. A multidisciplinary heart team is imperative to discuss the eligibility for TTVR, preprocedural planning, and post-procedure follow-up.
Technical Considerations
The anatomical factors of the TV apparatus, the venous system (IVC and superior vena cava), and their orientation play a vital role in the TTVR procedure. TTVR requires a large caliber sheath (up to 45 Fr) and delivery system compared with TTVr, as the tricuspid annulus is larger than the mitral annulus, and the tricuspid prosthesis needs to be even larger in cases with RV dilatation.22,32 Hence, TTVR is challenging in patients with small veins. The preferred access routes are transjugular and transfemoral. Transjugular access offers the advantage of having better alignment with the delivery system, but requires a large venotomy. Transfemoral access is safer, but the angle between IVC and TV is acute and requires extra maneuvering for successful valve deployment. The presence of a prior IVC filter may rule out a transfemoral approach. Transatrial access is an alternative approach. However, this approach requires minimally invasive right surgical thoracotomy. Ultimately, the site preference depends on operator expertise, institutional policy, and patient factors.
Valve positioning and anchoring are important technical steps in TTVR. Unlike the mitral valve, TV is not usually calcified. It may have implications for the anchorage of the prosthetic valve at the appropriate position and difficulties with fluoroscopic guidance. In particular, valve anchorage could pose serious challenges with an enlarged RV/tricuspid annulus. Choosing an appropriate-sized valve is key to a successful procedure. For this reason, self-expanding valves may be valuable to provide additional stability.22 For instance, the EVOQUE valve, available in sizes 44, 48, and 52 mm with a self-expanding nitinol frame, could be beneficial for patients with large annuli.20,32 Due to the proximity of the tricuspid annulus with the atrioventricular node, there is a risk of conduction abnormalities, such as high-degree atrioventricular block with TTVR. In the TRISCEND study, 11% of patients required a permanent pacemaker within 30 days.25 Although this number appears to be high, the need for a permanent pacemaker is even higher (>20%) after surgical TVR.33,34
Bioprosthetic degeneration is a significant concern after surgical or transcatheter valve replacement. As TTVR is at its incipient stage, long-term data about prosthetic valve durability and clinical outcomes are lacking. The long-term data regarding the durability of transcatheter aortic valve replacement is reassuring, with a low incidence of valve degeneration within 5–10 years post-implantation, despite being at a high shear stress position.35,36 Due to low shear forces at the tricuspid area, one can expect a lower likelihood of TTVR valve degeneration. For the same reasons, there is an elevated risk of thrombus formation due to the low flow state on the right side of the heart. Prospective trials are needed to investigate these issues further. Surgical TV reoperation carries very high in-hospital mortality at up to 37%.37 Transcatheter tricuspid valve-in-valve replacement can be considered for degenerative surgical bioprosthesis. Some studies demonstrated the safety of transcatheter tricuspid valve-in-valve replacement after failed surgical bioprosthesis.38,39 Although data on transcatheter tricuspid valve-in-valve replacement after degeneration of TTVR valves are lacking, it may be plausible and clinical trials are required in this space.
Procedural Planning
Multimodality imaging is crucial for defining the TV anatomy and function, assessing the right atrium (RA) and RV-related hemodynamics, and left-sided pathologies. The TV apparatus is a 3D structure comprising an annulus, leaflets (anterior, posterior, and septal), and subvalvular structures. Recent studies have shown that TV is indeed tricuspid only in approximately 50% and has four leaflets in 40% of the population studied.40–42 The morphology of the TV apparatus changes throughout the cardiac cycle. Moreover, the tricuspid annulus anatomy is not as well defined as the mitral annulus. It is a functional unit marked by atrioventricular junction and basal attachment of tricuspid leaflets.43
Pre-procedural Planning
Each patient undergoing TTVR has a unique TV anatomy. Therefore, a comprehensive preprocedural evaluation is needed to find the best-suited prosthetic valve. TTE is the initial test of choice for the assessment of TR severity, TV structure, and RV size and function. However, it is difficult to visualize all the leaflets simultaneously with 2D echo. Obtaining multiple views (parasternal long axis, apical, and subcostal) can help integrate the findings. According to the American Society of Echocardiography, severe TR is described by: vena contracta width >0.7 cm; effective regurgitant orifice area >0.4 cm2; regurgitant volume ≥45 ml; large central jet >50% of the right atrium; dense, triangular continuous wave jet or sine wave pattern on spectral Doppler; and systolic reversal of hepatic vein flow.44
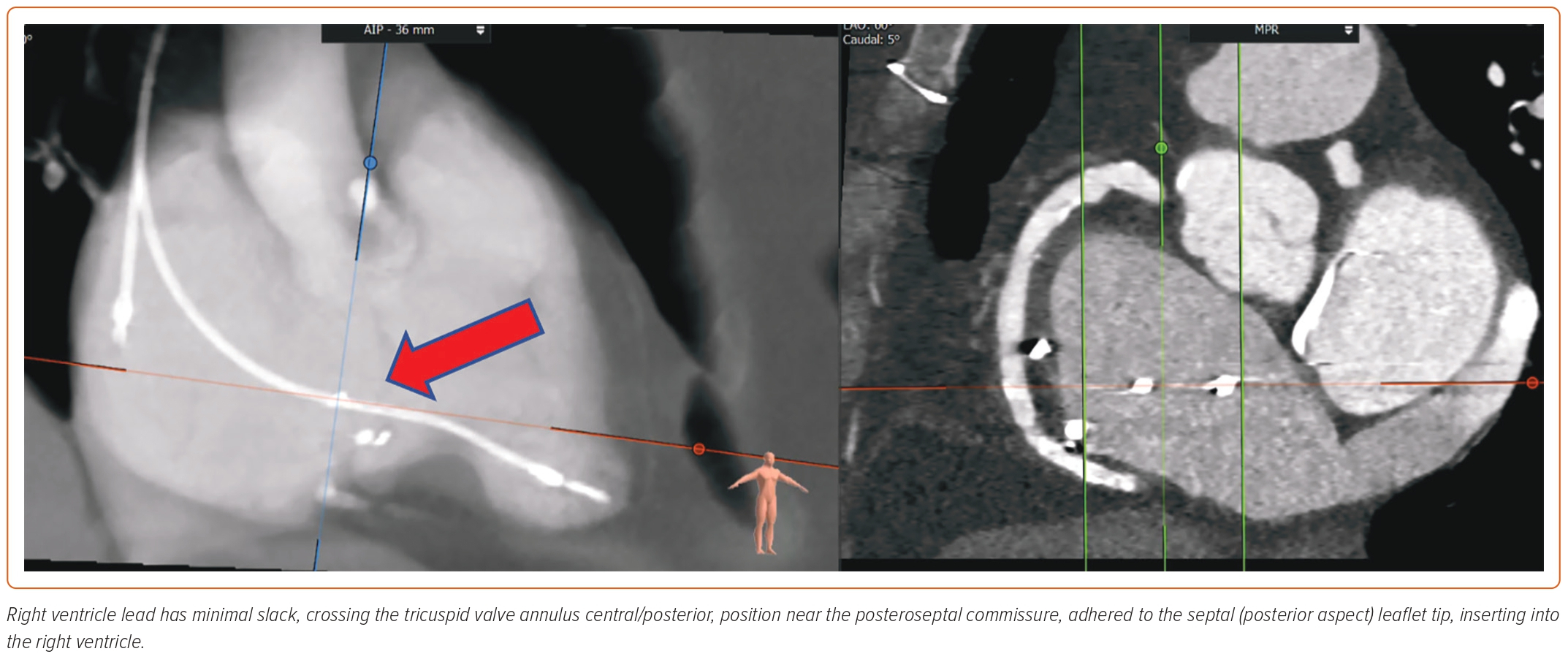
A new classification proposed by Hahn et al. further adds two more classes in severity, namely, massive (vena contracta 14–20 mm) and torrential (vena contracta ≥21 mm).45 RA and RV size, and hemodynamic data are vital to assess. Tricuspid annular plane systolic excursion in M-mode and tissue Doppler of RV free wall are helpful to determine RV function. Tricuspid annular plane systolic excursion <17 mm, fractional area change <35%, and tissue Doppler S’ <9.5 cm/s are classical markers of RV dysfunction.46 Novel parameters, such as 3D RV ejection fraction and 3D RV morphology, and RV free wall strain, are more reliable measurements that can now be obtained on TTE.47 TTE can also provide an estimate of pHTN, although right heart catheterization is often required for more accurate assessment in severe TR cases.48 Transesophageal echocardiography (TEE) is essential to accurately obtain the details of TV annulus and leaflets, and the mechanism of TR. Using biplane and 3D construction of TV apparatus, TEE is valuable in preprocedural planning. A thorough assessment by mid- and deep-esophageal and transgastric views is warranted. The 3D planar cross-sectional area of the tricuspid annulus should be measured in early systole and mid-diastole.43 Tricuspid annulus and RV size can be impacted because of volume overload. It is thus essential to perform measurements after volume optimization and close to the procedure date.49 Many patients undergoing TTVR have a transtricuspid pacemaker lead that requires a thorough evaluation of lead position in reference to TV leaflets. 3D imaging is valuable in determining the position of leads. The complications of TTVR include lead fracture or dislodgement. Often TTVR can be safely performed by entrapping the RV lead between native and prosthetic valves. However, lead extraction or conversion to epicardial leads may be required in select patients.30
CT angiography (CTA) scan is used in conjunction with echocardiography and has become a standard of practice for TTVR preprocedural planning. The benefit of CTA is twofold. It helps define the anatomy of the TV apparatus, RV, RA, and the blood vessels (venography). The images of the internal jugular, femoral, and subclavian veins are obtained to delineate feasible access sites. The angle between TV annulus IVC/superior vena cava is measured to plan for coaxial deployment of the valve system. An important consideration of TTVR is to avoid impinging on the right coronary artery (RCA) or coronary sinus. CTA can also help opacify the left heart and RCA, thus providing anatomical landmarks to avoid these structures.50 Optimal timing of contrast injection, such as using the pulmonary artery as the trigger for contrast bolus, image acquisition, heart rate control, and breath holding, are necessary for high-quality imaging. CTA can also mark RV size, trabeculations, papillary muscles, and moderator band, which can potentially interfere with the device delivery system. The RA should be assessed for optimal length to accommodate the device delivery system and ensure coaxial alignment.30 The drawback of CTA is the use of contrast, which may not be feasible in patients with advanced renal insufficiency. Lack of calcifications and poorly defined tricuspid annular anatomy make fluoroscopic guidance challenging.
Another important tool is cardiac MRI. It is a gold standard for quantitatively assessing cardiac structure and function due to its high spatial resolution.51 It is not just about the RV function, but its volume, shape, pattern of contractility (such as type II), and quality of myocardium, which could be derived from cardiac MRI.52 However, cardiac MRI may be contraindicated in certain situations, such as intracardiac devices or skeletal prostheses.
A preprocedural electrocardiogram should be obtained for all patients to investigate conduction abnormalities and risk stratification for the need for a pacemaker during or after TTVR. Dental screening is necessary, just like any other valvular intervention.53
Intraprocedural Imaging
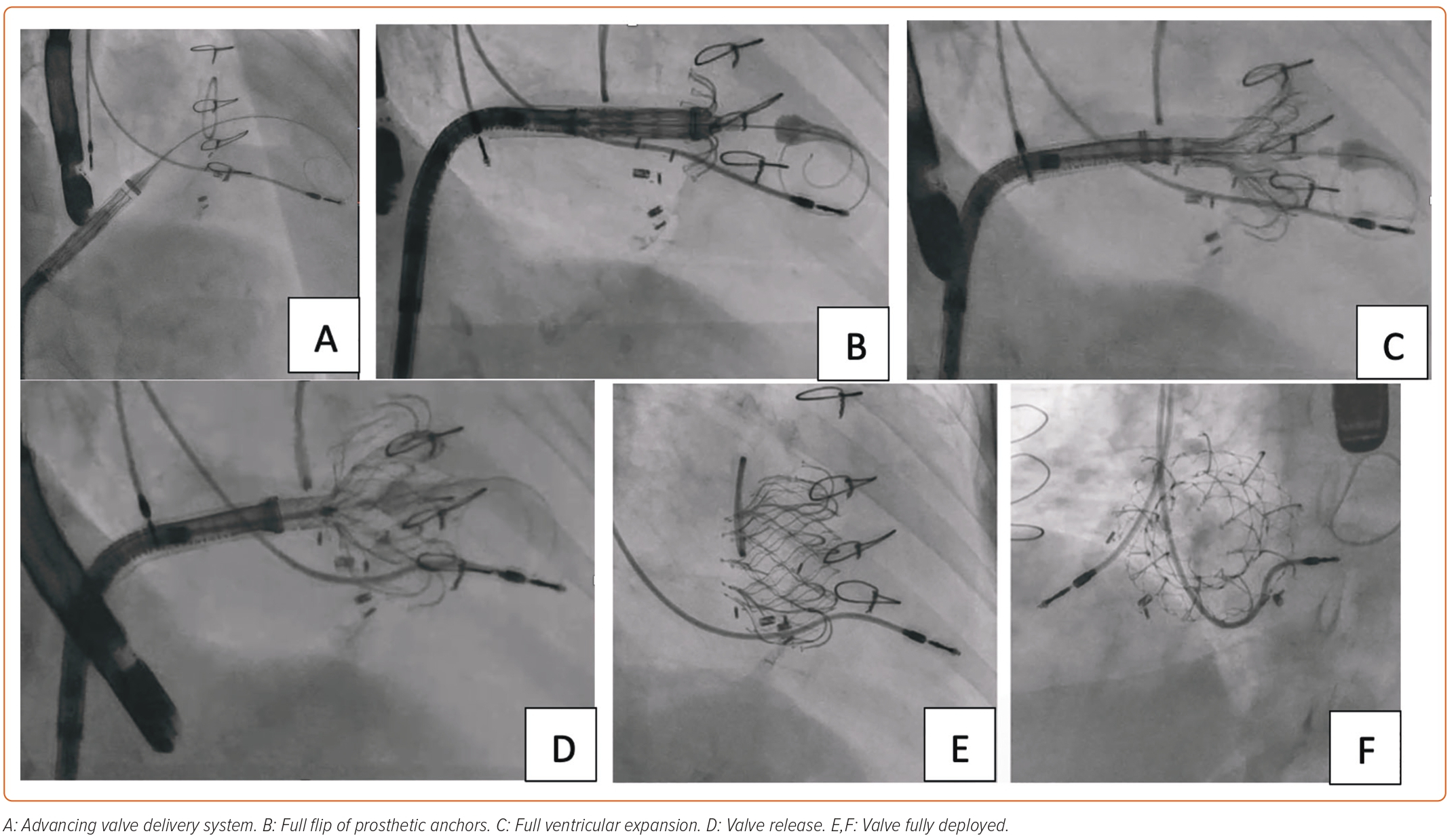
TTVR requires effective intraprocedural guidance. Multimodality imaging is fundamental to procedural success. 2D and 3D TEE using biplane views are essential for identifying spatial landmarks, including tricuspid annulus. TTE can be performed for intraprocedural monitoring if TV is not well visualized on TEE due to its anterior position. 3D intracardiac echocardiography is now being integrated into TEE/TTE for correct identification of the parts of the TV apparatus and navigation of the replacement device in cardiac chambers.54 The delivery system is advanced from the venous system to right heart chambers under fluoroscopic guidance. Simultaneous angiography of the RCA can provide an anatomical relationship of RCA to the tricuspid annulus. A guidewire can be placed in RCA as a marker.22,55 RCA runs in close proximity to the tricuspid annulus, which is an ill-defined structure. RCA injury must be avoided by carefully selecting the target zone. 3D TEE and fluoroscopy from multiple angles can help adjust the position of the delivery system and reach the target site. After prosthetic valve deployment, intraprocedural measurement of TR by color and spectral Doppler can provide real-time information about procedural success.
Post-procedure Follow-up
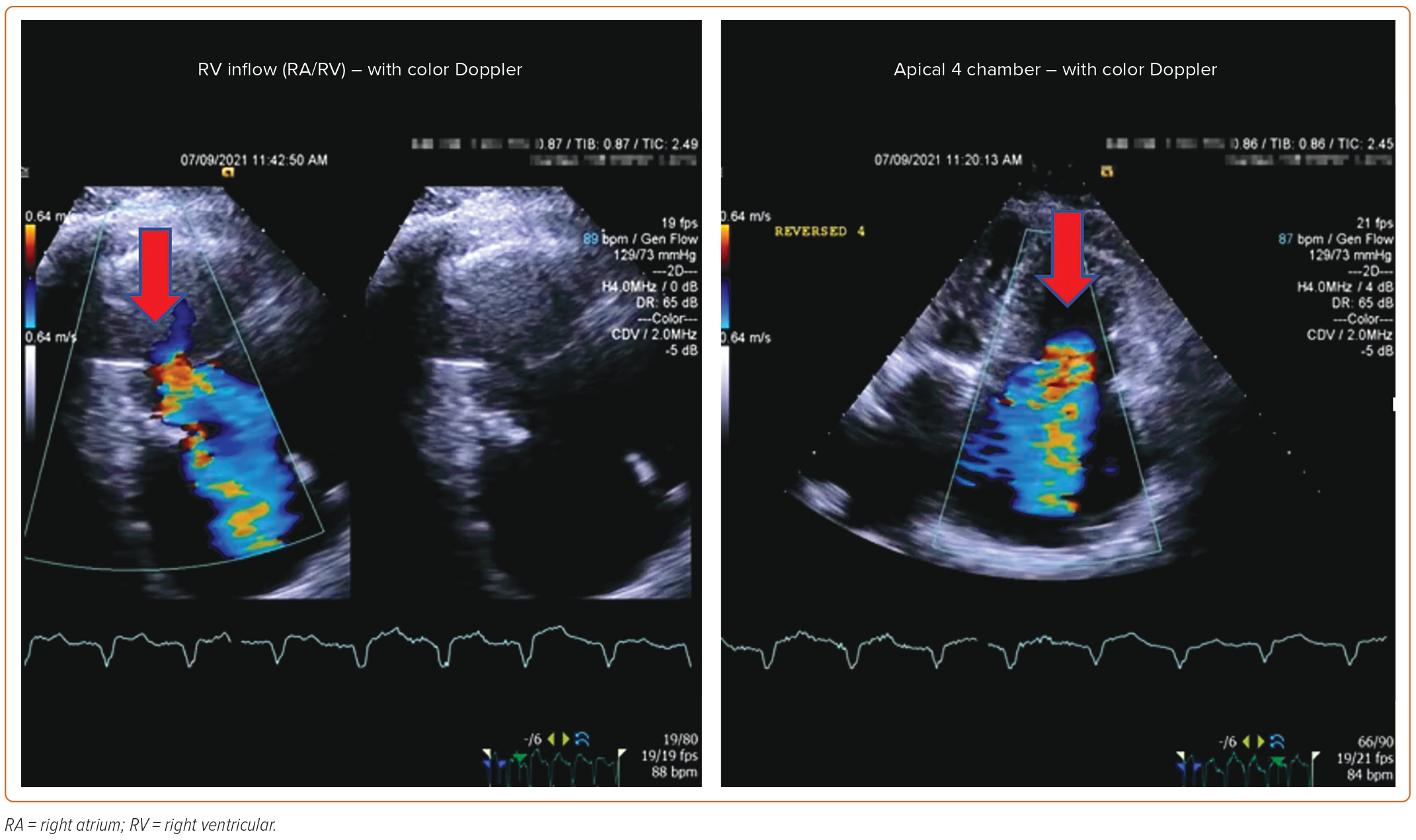
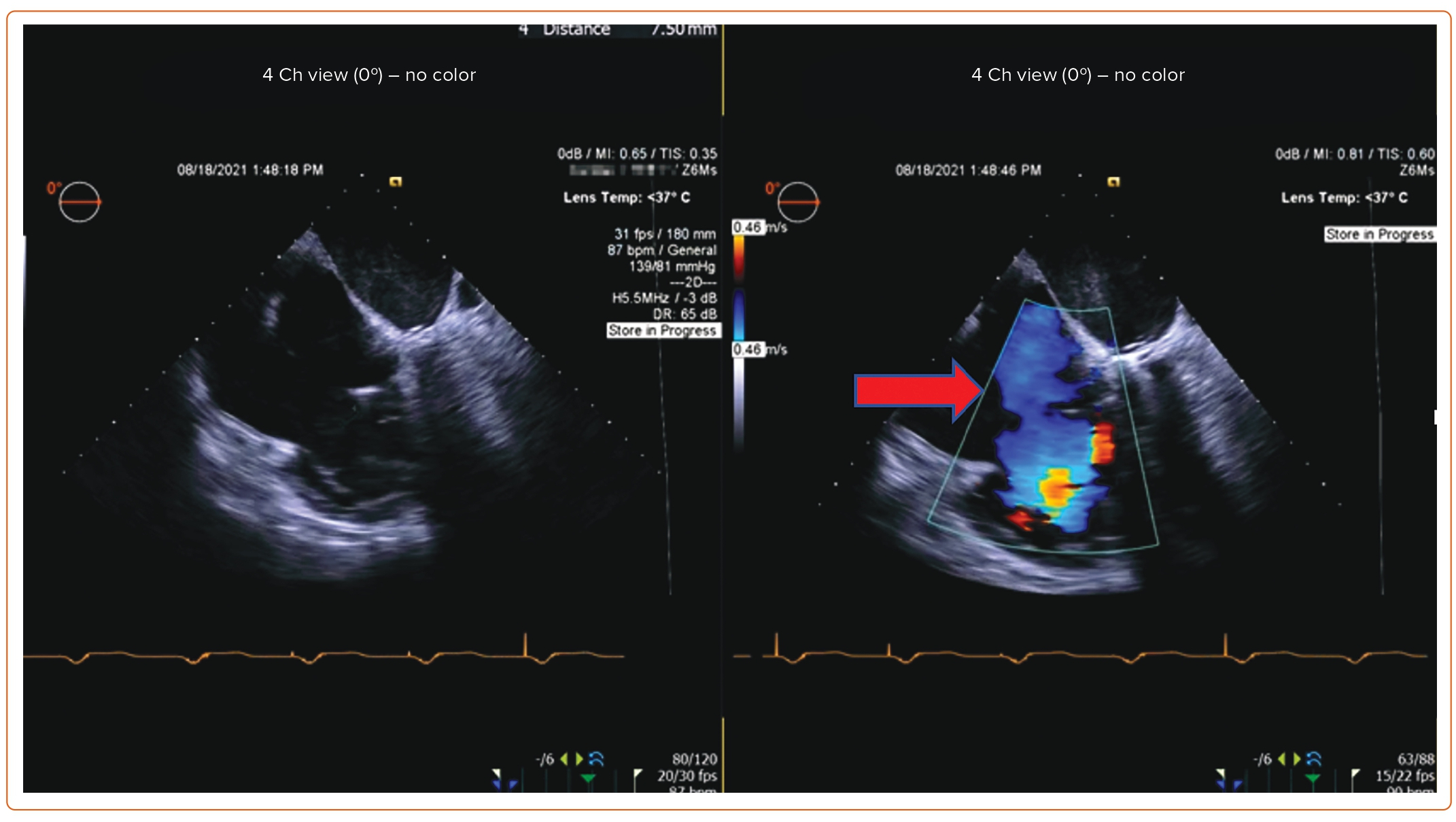
Post-procedure follow-up includes an assessment of clinical symptoms and of the severity of TR via TTE at 1 month and 1 year. If a device-related complication is suspected, such as thrombus formation, dislodgement of the device, or complete heart block, TEE can be considered.
Case Presentation
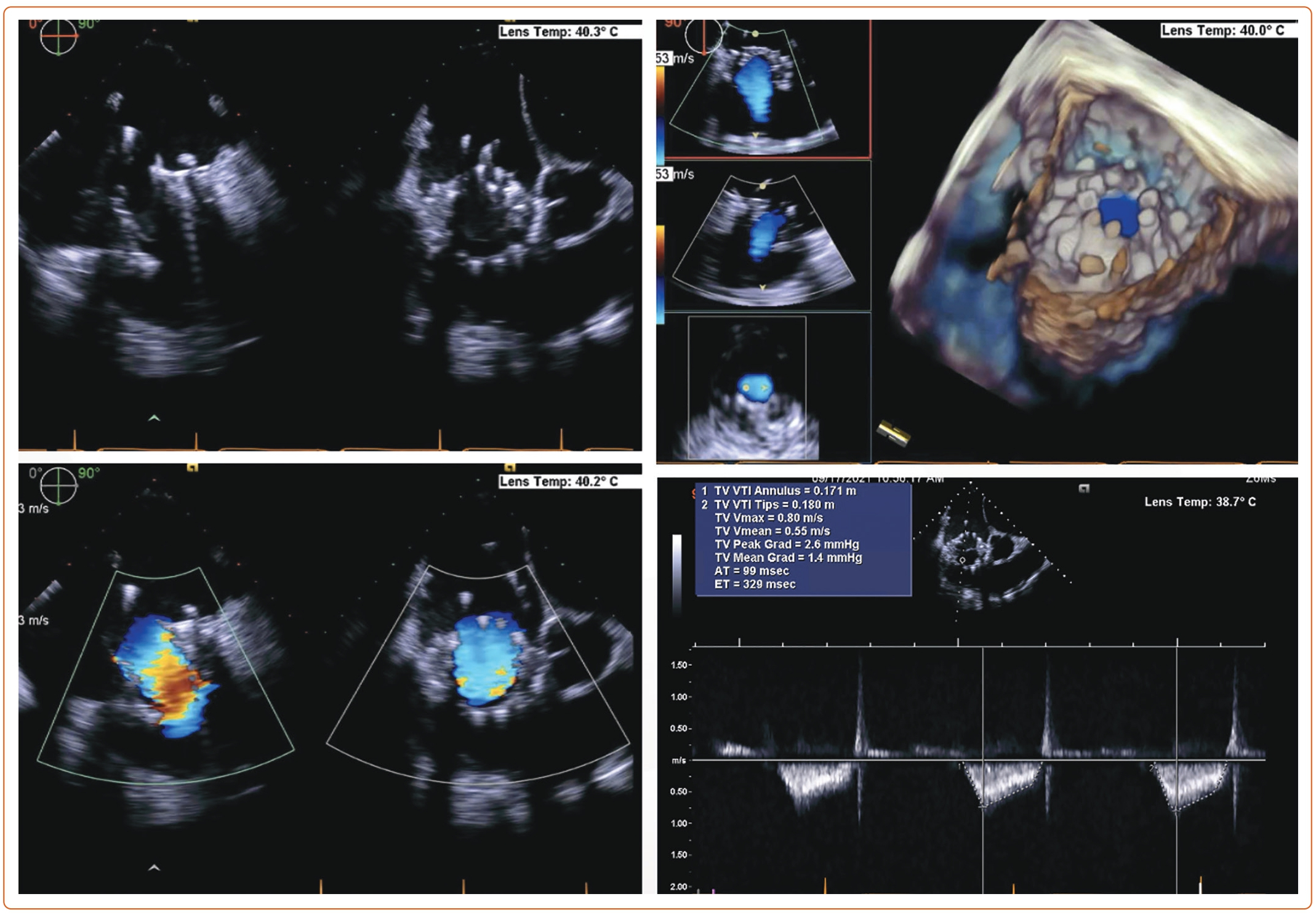
An 85-year-old woman with a history of surgical mitral annuloplasty, Trialign tricuspid repair 5 years earlier, AF, hypertension, chronic kidney disease, mild pHTN (PASP 40 mmHg), single-chamber pacemaker, and severe TR presented with dyspnea (New York Heart Association class III). Vital signs were stable. BMI was 32 kg/m2. She was chronically on warfarin therapy. Her STS risk score for mortality was 5.8%. The decision was made to pursue TTVR. The preoperative planning, intraprocedural images, and postprocedural follow-up images are shown in Figures 1–5.
Valves Used for TTVR
There are several TTVR valves undergoing clinical trials. Each one is designed with a unique anchoring mechanism, available valve sizes, and access site (Supplementary Material Table 1).
The EVOQUE System
The EVOQUE (Edwards Lifesciences) system comprises a nitinol frame, bovine pericardial leaflets, intra-annular fabric sealing skirt, and ventricular anchors (Supplementary Material Figure 1). It is delivered via transfemoral access using a 28 Fr delivery system, and is available in 44 mm and 48 mm sizes. The delivery system has a multiplanar steerable catheter, which allows coaxial positioning of the valve. Compassionate studies of TTVR using the EVOQUE valve have shown high procedural success, with clinical improvement and an acceptable safety profile.20 The TRISCEND trial, a multicenter, prospective single-arm study evaluating the safety and efficacy of the EVOQUE valve, showed technical feasibility, acceptable safety, significant TR reduction, and symptomatic improvement at 30-day and 6-month follow-up.25,56 One-year outcomes of the trial showed a high survival rate of 90%, high freedom from heart failure hospitalizations at 88.4%, and a sustained TR reduction with 97.6% of patients with mild or trace TR.57 The TRISCEND II Pivotal Trial is actively enrolling patients to compare the efficacy of TTVR using the EVOQUE valve with optimal medical therapy versus optimal medical therapy alone (NCT04482062).
The NaviGate System
The NaviGate valve (NaviGate Cardiac Structures) is a novel self-expanding device comprising a nitinol alloy stent with a tri-leaflet structure developed from equine pericardium (Supplementary Material Figure 2). The valve is designed to be delivered via the transjugular or transatrial approach, and is available in sizes ranging from 40 to 52 mm. The delivery system is inserted using a 42 Fr introducer sheath, and has a distal capsule that encloses the stent.58 The NaviGate valve was first implanted in 2017 in two patients for severely dilated tricuspid annulus and failed annuloplasty, both with high surgical risk.58 A single-center compassionate experience in five patients with the NaviGate device demonstrated a reduction of TR to ≤2+.19,49 All patients had a high burden of comorbidities. Patients with the transatrial approach had higher bleeding complications. One of the patients died on day 28. The remaining patients had clinical improvement associated with RV modeling and increased forward cardiac output.
LuX-valve
The LuX-valve (Jenscare Biotechnology) consists of a self-expanding nitinol stent with an atrial disc, interventricular septal anchor, and two graspers (Supplementary Material Figure 3). The prosthesis has annulus sizes of 50, 60, and 70 mm, with 26 mm and 28 mm valve sizes. It has been actively investigated in TRAVEL (NCT04436653) and TRAVEL II (NCT05194423) trials in China, and is delivered by the transatrial (TRAVEL) or transjugular (TRAVEL II) approach using a 32 Fr system. A compassionate multicenter trial showed procedural feasibility and low complication rates.26,59
Intrepid Valve
The Intrepid valve (Medtronic) comprises bovine leaflets within a nitinol self-expanding stent (Supplementary Material Figure 4). The delivery system of the Intrepid valve is unique due to its ability to recapture and retrieve the valve.60 It is currently being investigated in a TTVR early feasibility study (NCT04433065).
Cardiovalve
The Cardiovalve (Cardiovalve) was designed for transcatheter mitral valve replacement via a transseptal approach.61 It is now expected to have early feasibility trials with Cardiovalve for TTVR (NCT04100720).
Although TTVR is an exciting treatment option for patients who are not candidates for either surgery or TTVr, there are several limitations. Patients with severe RV or left ventricular dysfunction are excluded from ongoing trials. Patients with extreme dilatation of tricuspid annuli may not be amenable to TTVR due to the limited availability of prosthetic valve sizes and the need for a larger sheath. The need for anticoagulation adds yet another challenge and may exclude patients at high risk of bleeding.
Conclusion
Severe TR carries a poor prognosis when left untreated. Medical management may alleviate symptoms of RHF, but does not alter the pathology of TR. Isolated TV surgery for severe TR carries a poor prognosis, and several patients are ineligible for surgery due to high or inoperable risk. Such patients may be considered for transcatheter TV therapies, including TTVr or TTVR. Patients with large coaptation gaps, severe prolapse, tricuspid stenosis, and pacemaker lead-related TR, among other conditions, may benefit more from TTVR. Careful patient selection and procedural planning by the heart team will remain essential to procedural and clinical success. Several valves have shown good technical success and clinical outcomes in compassionate use patients and single-arm studies. TTVR is currently in its infancy, but rapidly developing and progressing into becoming the mainstream therapy for all TR patients regardless of their surgical risk. The results of large-scale multicenter randomized trials are ongoing to evaluate the benefit of these valves compared with medical therapy alone in patients with severe TR.