Definitions and Classifications
A bifurcation lesion is a diseased segment in a major epicardial coronary artery adjacent to or including the ostium of a significant side branch (SB). The diverse anatomical (bifurcation angle, vessel diameters) and pathological (severity, locations, and lengths of the lesions) spectrum of coronary bifurcation lesions (CBLs), along with dynamic alterations (carina shift, plaque shift) during intervention are responsible for the difficulties in the treatment of CBLs.1–3
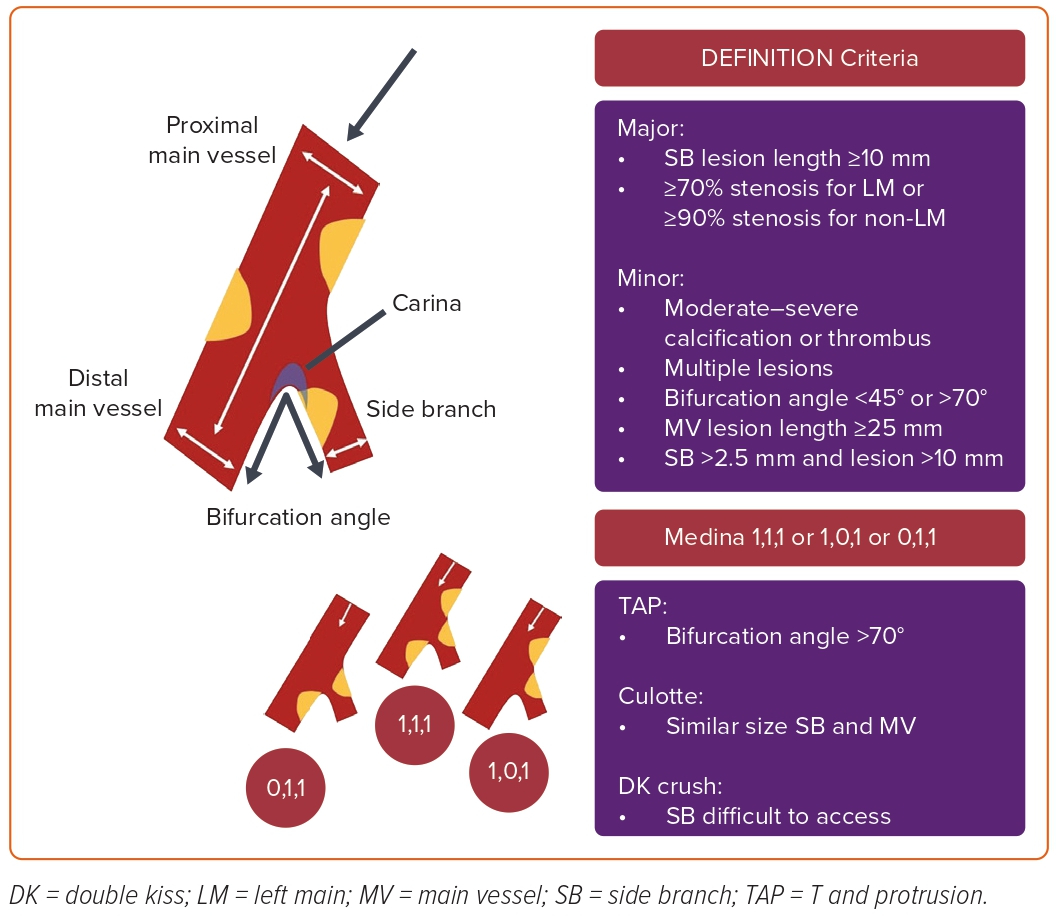
Bifurcations are among the sites with the highest predilection to atherosclerosis in the coronary arteries due to local flow disturbances and endothelial shear stress.4 Coronary arterial bifurcations show a fractal geometry pattern with a self-similarity principle.5 A coronary bifurcation contains a flow divider (carina region) and three vessel segments: the proximal main vessel (PMV), the distal main vessel (DMV), and the SB (Figure 1). There is a relationship between these three parts identified by Murray’s law as (diameter of PMV)3 = (diameter of DMV)3 + (diameter of SB)3.6 Angiographic measurements permitted the creation of Finet’s formula in normal human coronary arteries as the diameter of PMV = 0.678 × (diameter of DMV + diameter of SB).7 The polygon of confluence is the point where all three centerlines from the PMV, DMV, and SB meet. Finally, the bifurcation angle (carina angle) is essential in deciding the interventional strategy and accessing the SB.2,7 (Figure 1).
Various angiographic classifications of CBLs have been proposed. Because of its simplicity, the most widely used is the Medina classification.8,9 This classification is based on the existence (‘1’) or absence (‘0’) of a significant stenosis (≥50%) in the PMV, DMV, and SB of coronary bifurcations, respectively (Figure 1). Based on the Medina classification, a significant stenosis in both vessels (PMV and/or DMV and SB) is defined as true (Medina 1,1,1/1,0,1 and 0,1,1), while the rest are referred to as non-true.10 True CBLs are more complex and challenging to treat than non-true CBLs. The Medina classification does not consider other important information that might influence interventional strategies, such as lesion size and length, calcification, and bifurcation angle.9,10
The DEFINITION study defined complex bifurcation lesions (Medina 1,1,1/0,1,1 with SB diameter >2.5 mm) with the major and minor criteria depicted in Figure 1. Accordingly, a complex bifurcation is defined as having two major or one major criterion with two minor criteria.11 DEFINITION II was a randomized controlled trial (RCT) in patients undergoing percutaneous coronary intervention (PCI) for symptoms or proven ischemia with de novo complex bifurcation stenosis (Medina 1,1,1 or 0,1,1) as defined by the DEFINITION criteria. This study revealed that a two-stent technique is superior to a provisional stenting (PS) approach in true complex bifurcations.12
Differences between Left Main and Non-left Main Bifurcations
There are inherent differences between left main (LM) and non-LM bifurcations, as a result of which the approach to LM bifurcation PCI might differ from that of non-LM.1,13 The LM supplies >75% of the left ventricular myocardium, and the LM bifurcation is the only bifurcation where the proximal main bifurcation (MB) originates directly from the aorta; the implications of this include interaction with guide catheter, the potential for guide wires to go behind the LM stent, or for longitudinal stent compressions.1 Furthermore, although neither the left anterior descending artery nor the left circumflex artery (LCx) are strictly SBs, the consequences of occlusion or residual stenosis are clinically significant, perhaps more so than a non-LM bifurcation.1 Notwithstanding, the LCx is often the relevant SB, supplying >10% of the myocardium in >95% of cases; the LCx is also tortuous, adding to technical difficulty, and thus LM bifurcations differ in the aspects and detail to the attention required from that of an SB of non-LM bifurcation.13
Bifurcations of the LM have a wide (70–80°) and highly variable angle of separation between the two vessels. This T-shaped bifurcation angle of the LM may affect the implantation technique, and a highly angulated LCx take-off may impact the prognosis after LM stenting. LM bifurcations also often involve heavy calcification, and angiography alone is known to underestimate the extension of atherosclerosis in the LM, thus necessitating intravascular imaging to guide PCI.1,13 This review focuses explicitly on the contemporary approach to non-LM bifurcation lesions, which typically do not ascribe to the aforementioned complexities of LM bifurcations. However, non-LM lesions also have their unique challenges. These include small SBs, which are often not amenable to stenting hence limiting the operator to a single stent strategy;14 extreme tortuosity, which could render the delivery of stents more difficult; multiple SBs, which further complicate the choice of strategy; and long diseased segments, which make lesion preparation and identification of suitable landing zones across the SB a particular concern.
Deciding on an Optimal Strategy
While assessing CBLs and deciding the optimal PCI strategy, an integrated approach, including several important considerations, should be adopted. Besides the above-mentioned anatomical definitions, patients’ characteristics, cath lab equipment, lesion characteristics, operator expertise, and procedural aspects should be meticulously evaluated to improve the short- and long-term outcomes. Regardless of the approach, one should understand the original bifurcation anatomy and try to achieve an optimal flow in both vessels while limiting the metallic stent coating.
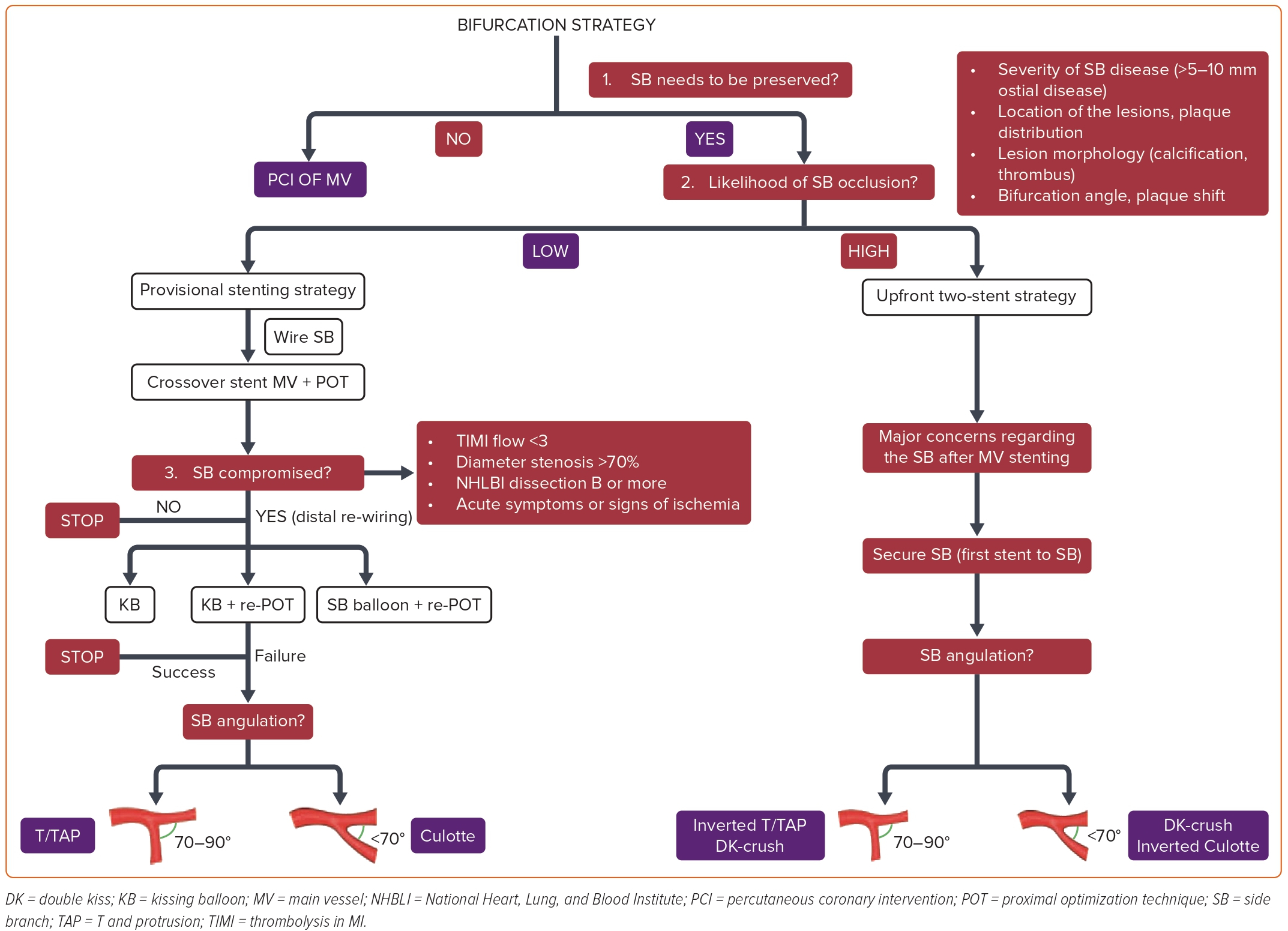
Figure 2 summarizes an algorithmic approach to finding the optimal PCI method for patients with CBLs. The first step of this algorithm is to decide whether or not the SB should be preserved. If not, this is not a true bifurcation lesion, and the MV can be stented without advancing a second guidewire to the SB. In contrast, an SB that deserves to be preserved means that the loss of relevant SB flow may lead to ischemic symptoms and reduced left ventricular ejection fraction because of the compromised blood flow of a significant amount of myocardial mass (>10%). It is crucial to remember that only 20% of non-LM SBs supply >10% of the myocardium.13 If a decision is made to preserve the SB, a guidewire should be placed in the SB, and the most crucial step of the algorithm, which is the likelihood of SB occlusion, should be evaluated. Besides the anatomical location of the lesions defined by the Medina classification, the likelihood of the SB flow compromise depends on the severity and length of the SB disease, lesion morphology (calcification, thrombus), and bifurcation angle.8 Additionally, to quantify the risk of SB occlusion after MV stenting, the V-RESOLVE scoring system was introduced.15 Greater proximal MV stenosis, MV plaque distribution ipsilateral to the SB, reduced MV thrombolysis in myocardial infarction (TIMI) flow before stenting, a bifurcation angle >70°, a larger MV:SB ratio, and greater SB stenosis were identified as the six independent predictors of SB occlusion.
Severity (SB diameter stenosis ≥70%) and length (≥10 mm) of the SB disease, amount of calcification, and bifurcation angle (<45° or >70°) are among the essential parameters to consider while anticipating the risk of SB loss, and already included in DEFINITION criteria used to define complex CBLs (Figure 1).11
The risk of acute SB occlusion during MV stenting is around 8% and is closely related to the increased risk of MI and cardiac death.16 One of the proposed mechanisms for compromised SB flow after crossover stenting is carinal shift, caused by an overexpanded stent in the distal MV and is more frequently encountered in acute angled and small diameter SBs. At this point, it is crucial to bear in mind that – despite the critical angiographic appearance – most of the carinal shifts limited to the SB ostium do not lead to significant physiological changes throughout the vessel.17 Plaque shift is the second and less common reason for SB occlusion or stenosis after MV stenting. Intracoronary imaging studies have demonstrated that the decrease in the proximal MV plaque volume after MV stenting is associated with the plaque shift to the SB ostium, a finding that supports the close relationship between the severity of proximal MV disease and SB flow compromise.18
As in non-bifurcation territories, the presence of calcium in bifurcation lesions makes the PCI more challenging and is associated with worse outcomes.19 An optical coherence tomography (OCT) study by Fujino et al. documented that more than mild calcification at the bifurcation site portends a high risk of SB flow deterioration after MV stenting.20 Beyond plaque modification by cutting or scoring balloons, cracking the calcium with dedicated devices (rotational or orbital atherectomy or intravascular lithotripsy) is recommended to prepare calcified CBLs. Considering the shortage of angiography in evaluating calcium burden, intravascular imaging should be implemented into daily practice to assess the plaque distribution, proper vessel size, and the extent of calcium in CBLs.
The bifurcation angle is another essential determinant to anticipate SB occlusion risk. The carina shift and coverage of the ostium will not pose a significant issue at wide bifurcation angles. More acute bifurcation angles are prone to a higher risk of carina shift, and it is challenging to cover the SB ostium with a stent. However, rewiring and gear advancement to the SB are more straightforward. In contrast, widely angulated SBs can be challenging for the rewiring process and stent delivery, a consideration that influences the operator for upfront stenting into an SB at high risk of compromise.
Following a comprehensive assessment, if the likelihood of the SB occlusion is low, a stepwise provisional SB stenting approach should be preferred. Of note, it is essential to remember that a stepwise provisional SB stenting strategy does not always mean a single stent technique. In case it is needed, the provisional strategy allows for escalation to the bailout two-stent techniques. The stepwise provisional SB stenting approach begins with wiring both vessels and deploying a crossover stent from the PMV to DMV, followed by the proximal optimization technique (POT) with a non-compliant (NC) balloon sized 1:1 to the PMV diameter. As depicted in Figure 2, after rewiring from the distal stent strut, kissing balloon inflation (KBI), KBI plus re-POT or SB balloon inflation plus re-POT are the options to optimize SB flow in case of persisting SB flow compromise, National Heart, Lung, and Blood Institute C to F dissection, or signs of ischemia after MV stenting. If these approaches fail to achieve an acceptable result in the SB flow, a second stent should be deployed using T, T and protrusion (TAP) or culotte techniques according to the bifurcation angle.
In contrast, if the likelihood of SB occlusion after MV stenting is anticipated to be high, a systematic (upfront, planned) two-stent strategy should be applied. These bifurcation lesions are generally identified as complex bifurcations in which a long lesion in the SB makes access (rewiring) and stent placement complicated following MV stenting. In such bifurcation anatomy, where there is a major concern regarding the SB after MV stenting, the SB should be stented first by using an inverted T/TAP, inverted culotte, or double kissing (DK) crush technique following the bifurcation angle, a strategy allowing the SB flow to be secured. While inverted T/TAP stenting is recommended for the bifurcation angles between 70° and 90°, inverted culotte or DK crush stenting is the preferred approach in more acute bifurcation angles (<70°). Beyond operator expertise, the diameter correlation between DMV and SB becomes more of an issue for the latter. DK crush stands as a rational approach in cases with a significant diameter gap between the MV and SB. Concerning the safe expansion limits of the stents deployed, such a diameter difference may limit the withdrawal of the SB stent to the PMV, thus applying the inverted culotte. However, in cases with similar diameters of DMV and SB, the inverted culotte technique using a safely expanding stent up to the PMV diameter following POT is a plausible option.
Current Evidence for Each Strategy
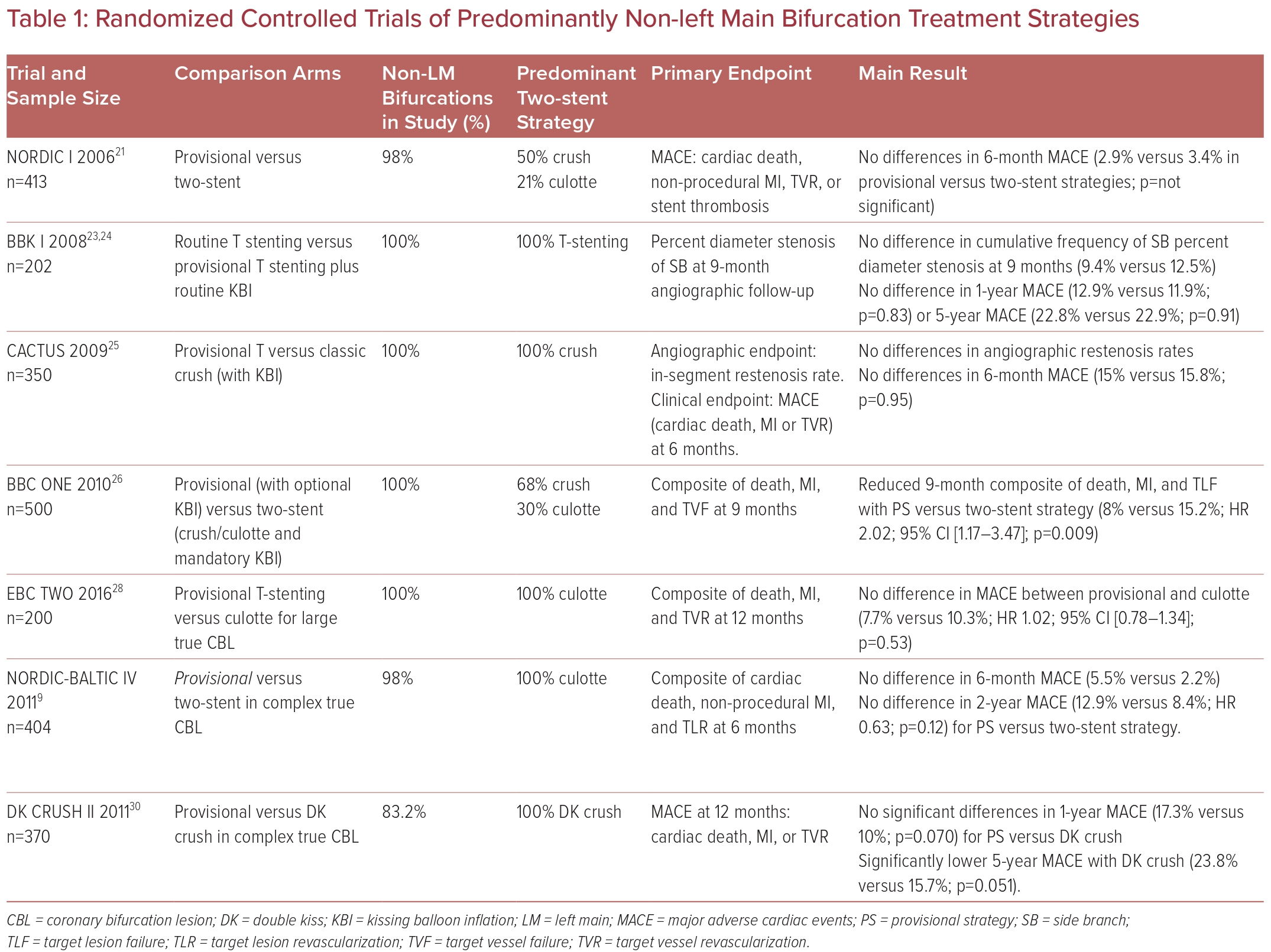
The current recommendations of a provisional strategy as default for bifurcation lesions largely emerge from RCT evidence, comparing PS versus various two-stent strategies (Table 1), incorporating multiple iterations over the years. As with procedural trials, operator heterogeneity remains, particularly in terms of personal preference, technical variations of the steps, and geographic prevalence in practice, which is particularly pertinent to bifurcation PCI. Notably, observational studies are prone to bias, as an upfront two-stent strategy is invariably preferred in true complex CBLs.
The Nordic I bifurcation trial, the first randomized trial comparing an upfront two-stent with PS strategy, found no differences in 6-month major adverse cardiac events (MACE) (3.4% versus 2.9%), with only 4% of the PS arm proceeding to SB stenting.21 All bifurcation types were included, 2% were LM bifurcations, and the crush technique (50%) was predominantly used in the two-stent arm. At 5 years, there remained no differences in mortality across the arms.22
The BBK trial randomized 202 patients to routine T-stenting versus to provisional T-stenting and routine KBI.23 Nineteen percent of PS patients proceeded to T-stenting. There were no differences in the primary angiographic endpoint, nor 1-year or 5-year MACE.24
Colombo et al. reported the CACTUS trial results the following year, which randomized 350 patients with true bifurcation lesions to the classic crush strategy versus PS with mandatory KBI.25 In total, 94% of patients had true bifurcation disease. Six-month angiographic restenosis and MACE (15.8% versus 15%; p=0.95) were similar.
The large BBC ONE trial randomized 500 patients to either PS with optional KBI or a systematic two-stent strategy (crush/culotte) with mandatory KBI.26 Eighty-two percent had true bifurcation lesions. Both in-hospital (8% versus 2%; p=0.002) and 9-month MACE (15.2% versus 8.0%; p=0.009) were significantly higher with an upfront two-stent strategy, driven by excess MI, indicating that the provisional technique is the preferred strategy.
Five-year pooled patient-level analysis combining outcomes from the two largest trials comparing PS versus two-stent strategies (Nordic I and BBC ONE) further confirmed lower mortality with the PS compared with systematic dual-stenting (3.8% versus 7%; p=0.04).27
Subsequent trials evaluated PS against various two-stent techniques for more complex bifurcation lesions. The EBC TWO randomized trial compared PS versus culotte exclusively in 200 complex true bifurcation lesions with large SBs with significant ostial disease length.28 PS remained similarly effective in complex CBL versus culotte at 12-month MACE (7.7% versus 10.3%; p=0.53). The Nordic-Baltic Bifurcation Study IV, which also randomized patients with true complex bifurcation disease, including SB disease length of 15 mm, found no differences in 6-month and 2-year MACE, nor any differences in the improvement of angina.29
DK CRUSH-II compared PS to the elaborate DK crush technique in 370 Asian patients with complex bifurcation lesions.30 Although the 12-month MACE was not different between the groups, target vessel revascularization (TVR) was significantly reduced with DK-crush (6.5% versus 14.6%; p=0.017), with similarly reduced target lesion revascularization (TLR) at 5 years driving a reduction in 5-year MACE for the DK crush arm.30
A frequentist network meta-analysis of 14 studies, including 4,285 patients from bifurcation RCTs, found that DK crush and mini-crush were associated with significant reductions in MACE, TVR, and TLR compared with PS (RR 0.31−0.55 [all p<0.01] and RR 0.42–0.45 [all p<0.02], respectively) and other bifurcation techniques. Culotte and crush techniques had a higher risk for stent thrombosis compared with PS.31
Notably, the majority of the early randomized studies comparing PS to elective two-stenting used a first-generation drug-eluting stent (DES). It is important to note that the improvement of the stent platform of the current-generation DES (in terms of strut thickness, SB expandability, maximal stent expansion capacity) might contribute to similar clinical outcomes between provisional versus two-stent strategies, unlike first-generation DES, where provisional strategies appeared to have better outcomes.32
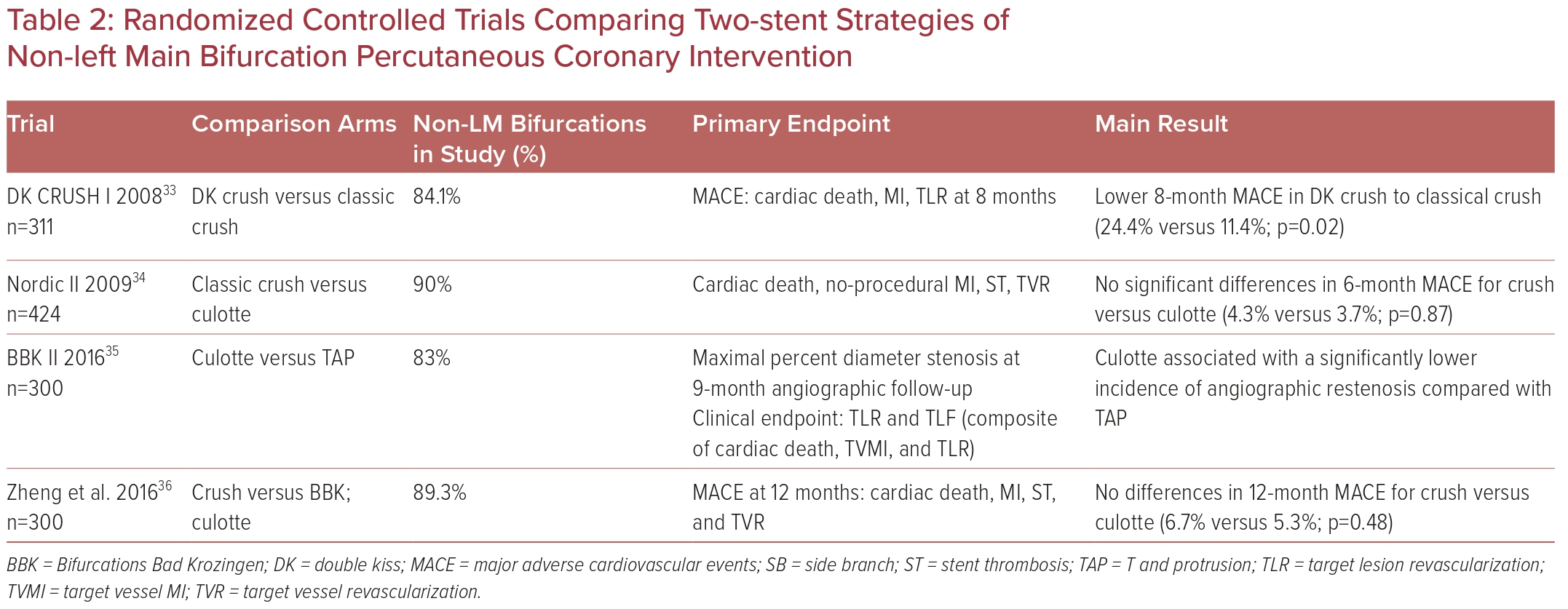
Different upfront two-stent strategies have also been evaluated to a limited extent in RCTs (Table 2).33–36
Evaluation of Stenting Techniques
T/T and Protrusion Stenting
Initially, all the steps of the PS technique should be performed.1,37,38 Then, if the bifurcation angle is near 90°, positioning the second stent in a T configuration is feasible and allows stent strut coverage of the bifurcation.39 Generally, the angle is not 90° and this results in incomplete SB ostium scaffolding or stent protrusion inside the PMV. Limitations of T stenting ensued in the development of the TAP technique. The TAP technique creates a neocarina but achieves complete stent coverage of the SB ostium. The shortest neocarina with complete coverage of the SB ostium should be aimed for. Before deploying the SB stent, a balloon sized 1:1 to the DMV should be parked in the MV. After SB stent deployment, the balloon of the stent is slightly pulled back and reinflated at high pressure for optimal stent expansion at the SB ostium. Following KBI, the balloons should be deflated simultaneously to keep the neocarina centrally. A final POT above the neocarina is recommended to preclude the bottleneck effect in the PMV created by the long overlaps of the KBI balloons.1,37,38 However, meticulous attention should be given to performing the final POT above the neocarina to preclude crushing the neocarina towards the SB, or the final POT can be avoided when the proximal MV stent segment is too short to allow post-dilatation without carinal disturbance or proximal geographical miss.
Culotte Stenting
The culotte technique, which can be used either as a bailout or a systematic two-stent strategy, is generally preferred when the SB and DMV have similar diameters with a narrow (<70°) bifurcation angle. Culotte stenting is often performed by implanting the first stent in the SB, called the inverted culotte.7,39 After appropriate pre-dilation of each vessel, the first stent is implanted in the SB protruding inside the PMV. The stent is sized according to the SB diameter and long enough to allow the POT in the PMV. POT is performed using a balloon sized 1:1 according to PMV. The MV is rewired from a distal stent strut close to the carina. After the first KBI, a modification to preclude the first stent’s malapposition during the second stent deployment (first kiss of double kissing), an MV stent sized according to the DMV is deployed. Following the second POT, distal rewiring of the SB and second KBI with short NC balloons is performed. The procedure ends up with the final POT. Attention is required to select stent platforms that can be safely expanded to reach the PMV size. Accordingly, the culotte technique may not be optimal for CBLs with significantly different branch diameters. The major limitation of the culotte technique is the overlapping stents in the carina and PMV.1,37,38 This limitation may be sorted out with the mini-culotte approach, which minimizes the overlapping stent segments in the PMV.40
Double-kissing Crush Stenting
DK crush stenting is generally the preferred option when there is a major concern regarding the SB, which is anticipated to be difficult to access after MV stenting. The DK crush stenting starts with optimal pre-dilation of both branches, parking an NC balloon sized 1:1 to the DMV and stenting the SB. The stent protrusion to the PMV should be limited to 2–3 mm. The SB stent is inflated while the MV balloon is kept uninflated in the MV. After SB stent deployment, the balloon of the SB stent should be slightly pulled back and repeated inflation at high pressure should be performed for proper apposition at the SB ostium. Following SB balloon and guidewire removal, protruded SB stent struts are crushed in two steps. After crushing stent struts with an NC balloon sized 1:1 to the DMV, which is already parked at the MV, the POT crush with an NC balloon sized 1:1 to the PMV should be performed to complete crush and ease rewiring the SB from a non-distal stent strut, which the first KBI follows. After removing both balloons and SB guidewire, a stent is deployed in the MV (sized 1:1 with DMV). POT is performed, and the SB is rewired through a non-distal stent strut. The procedure ends with the second KBI and the final POT.38,41–43
Proximal Optimization Technique
The MV stent is usually sized according to the DMV to minimize the carina shift. This leads to some degree of malapposition in the PMV as per Murray’s law, which is mitigated by the POT.13 POT is recommended routinely after MV stenting by the European Bifurcation Club, irrespective of the stenting strategy.44 First devised by Olivier Darremont, POT entails the expansion of the PMV stent by a short NC balloon inflation, sized 1:1 according to the PMV.45 PMV stent length selection must be made to accommodate the shortest available POT balloon on the shelf. The balloon must be meticulously positioned, with its distal shoulder accurately placed, immediately proximal to the carina, bearing in mind manufacturers’ varying marker-to-shoulder distances of balloons.46 This can be technically challenging; thus, multiple radiographic views and stent enhancement techniques may be helpful.44
The POT balloon should also afford good proximal stent coverage, ideally just reaching the proximal stent edge in the MV.13 If the balloon extends too proximally beyond the stent, there is a risk of edge dissection. If the balloon is placed too distally, leading to incomplete coverage of the proximal stent edge, there is the risk of bottleneck stent malapposition. In such cases, the balloon should be repositioned and reinflated, ensuring full coverage and sufficient expansion of the proximal part of the stent. POT should be performed before SB rewiring.44 By expanding the MV stent proximal to the SB, POT facilitates SB rewiring, distal stent strut crossing, and reduces the risk of accidental abluminal rewiring.44,47 A repeat POT or re-POT is routinely recommended in two-stent strategies after KBI, with a POT balloon diameter sized 1:1 to PMV.39,48 Of note, the correct placement of POT balloons may differ according to the preferred two-stent strategy. In culotte stenting, the first and second POT should be performed immediately proximal to the carina after the first and second stent implantation, respectively. Nonetheless, the final (third) POT should be performed more proximal to the (neo)carina.38 Regarding DK crush stenting, the 16th expert consensus document of the European Bifurcation Club recommends two-step crushing of the SB stent. It refers to the second step as ‘POT crush,’ performed immediately proximal to the carina.38 Following MV stenting, the second POT should be performed immediately proximal to the carina, which is different from the final POT that should be performed above the centralized double-layer neocarina.38
The rationale for POT has been derived predominantly from bench studies with a lack of clinical data or validation.46,49 Recent data from the large e-ULTIMASTER registry reported significant reductions in 12-month TLF and stent thrombosis with POT in the bifurcation stenting cohort who had an already low rate of TLF at 1 year.50 However, despite solid rationale and recommendations for POT, the same registry reported a gross underuse of the technique (33.3%).50
Side Branch Treatment and Kissing Balloon Inflation
SB intervention after MV stenting largely depends on the SB status evaluated after PS. The keep-it-open (KIO) principle is adopted for an acceptable SB result. In KIO, both branches are wired, the MV is stented, and POT is performed, with no further intervention in the absence of flow reduction in the SB.13,44 In the absence of reduced TIMI flow, routine SB balloon dilatation is not recommended in PS.13 The evidence for KBI in non-LM bifurcation lesions varies, with only limited long-term data regarding routine KBI, particularly in non-LM bifurcation lesions. In the Korean COBIS registry, among non-LM bifurcation lesions with a PS, KBI was associated with a higher incidence of MACE and TLR, which remained significant after propensity score matching.51 In contrast, the Nordic-Baltic Bifurcation Study III, which randomized 477 patients with bifurcation lesions to final KBI versus no final KBI after MV stenting, found no difference in 6-month MACE in routine KBI.52 However, KBI reduced angiographic SB (re)stenosis, especially in patients with true bifurcation lesions.52 In the SMART STRATEGY RCT, 258 patients (of whom 55.8% had non-LM bifurcations) were randomized to one of two arms: a ‘conservative’ strategy (SB intervention if TIMI flow <3) or an ‘aggressive’ strategy (SB intervention if >75% SB stenosis). The SB intervention comprised SB ballooning, KBI, and stenting if needed. At 12 months, similar rates of target vessel failure (TLF) were seen (9.4% versus 9.2% in the conservative versus aggressive strategy, p=0.97);53 however, at 3 years, the conservative strategy had reduced target vessel failure (11.7% versus 20.8%; p=0.049), cardiac death, or MI (0.8% versus 6.2%; p=0.036).54
Thus, in PS of non-LM bifurcations, KBI is recommended only in the presence of TIMI flow <3 or acute signs or symptoms of ischemia.13 KBI is preferred over isolated SB balloon dilatation, which might displace stent struts opposite to the carina and compromise MB stent performance.55 However, in all two-stent strategies, KBI is a mandatory step and improves outcomes.32,39,56 To perform KBI, the SB is rewired through the distal stent strut after POT for the optimal opening of SB ostium and scaffolding.49,57–60 Short NC balloons should be used, which are resistant to deformation, overexpansion and, thus, dissection.61,62 Balloons should be sized 1:1 to distal reference diameters of respective vessels with minimal balloon overlap in PMV.39,63
A two-step ‘sequential’ KBI is recommended, beginning with alternate higher-pressure inflation of MV and SB, followed by lower-pressure simultaneous inflation and then simultaneous deflation, to centralize the neocarina while stents are opposed and retain this modified architecture.13,39,57,58 Nevertheless, as KBI causes elliptical distortion of the PMV stent, it should always be followed by a re-POT or final POT to prevent stent malapposition.63,48
POT/Side/POT
The POT/side/POT strategy was proposed and debated at the 14th European Bifurcation Club consensus meeting as an alternative to the POT/kiss/POT strategy, which can cause elliptical distortion of the PMB.44 Minimal material is used: initial POT is followed by SB crossing, SB dilatation with an NC or semi-compliant balloon, and completed by a final POT with the same initial POT balloon.64 POT/side/POT also enables slender bifurcation PCI through a smaller 5-Fr catheter, the feasibility of which was demonstrated in a study (n=80) by Sguegulia et al.65 Despite encouraging bench-testing and real-world results, this technique’s success depends on optimal POT and distal cell crossing, which can be challenging.66 Further, in contrast to the POT/kiss/POT strategy, where the final POT balloon is proximal to the carina, in the case of POT/side/POT, the final POT should cover the SB ostium, to rectify malapposition of the opposing stent wall caused by SB dilatation.13
Jailed Side Branch Wire
A jailed wire in the SB is recommended until POT is performed and the SB is rewired.44 A jailed wire in the SB was associated with flow recovery in the COBIS II registry.16 The wire also serves as a marker for the SB origin, facilitating SB rewiring and optimization of bifurcation geometry, and as a bailout in case of intricate SB rewiring by providing a track for a low-profile balloon crossing and dilatation.13,44
Jailed Balloon and Modified Jailed Balloon Technique
As an alternative to prevent acute SB occlusion, the novel jailed balloon technique (JBT) was reported by Burzotta et al. with various subsequent modifications described.67,68 In the JBT, an uninflated balloon in the SB remains jailed under the MV stent struts during MV stenting and positioned to overlap entirely with the MV stent. If the SB flow is preserved, the jailed balloon is removed uninflated. If the SB occludes after MV stenting, the jailed balloon can be inflated to restore SB flow or used as a marker for rewiring, similar to the jailed wire, followed by KBI if the jailed balloon was inflated.67
A modified JBT (MJBT) was proposed by Saito et al. with its short-term safety and efficacy in preserving SB patency for true bifurcation lesions reported.69,70 Here, a smaller SB JB (half the size of the MV stent) is placed so as not to overlap in MV. Both the stent and JB are simultaneously inflated with the same pressure, with the proximal end of the JB attaching to the MV stent. The JB is removed, and wires are recrossed, followed by KBI and TAP stenting if needed.71 In a small SB subgroup analysis of the CIT RESOLVE trial, among patients with a high risk of SB occlusion (V-RESOLVE score ≥12 points), JBT was superior to jailed wire technique in reducing SB occlusion.71 A systematic analysis of 908 patients from six studies comparing 615 conventional JBT versus 293 MJBTs found that MJBT was superior for MACE, with reduced SB loss and dissection.72
The Role of Intravascular Imaging
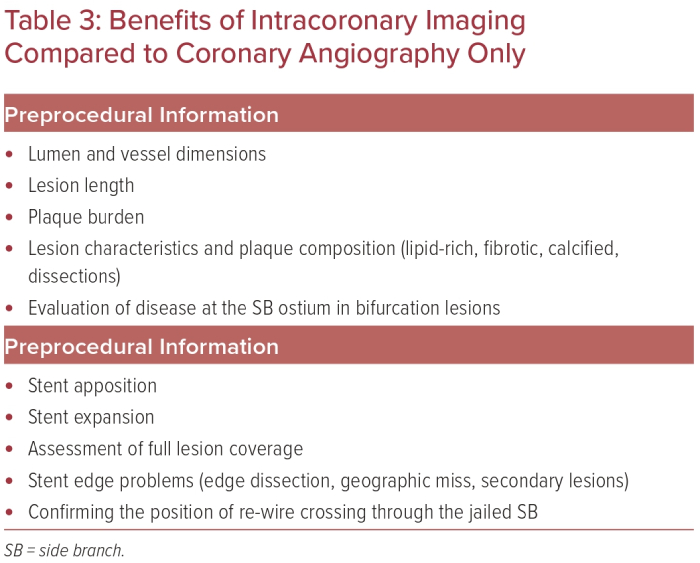
Intravascular ultrasound (IVUS) and OCT are the technologies most widely used worldwide for intracoronary imaging. IVUS has been used since the mid-1990s and has therefore achieved greater use worldwide than OCT, which became commercially available in the late 2000s. Both modalities provide valuable information compared with the 2D coronary angiogram pre- and post-PCI. This paragraph is intended to provide an overview of recent data and ongoing trials for intracoronary imaging for non-LM bifurcation lesions, in addition to the rational benefits of the technique shown in Table 3.
Data on Intravascular Ultrasound-guided Non-left Main Bifurcation Stenting
While no randomized control trials have been published specifically for bifurcation imaging-guided PCI, there are 3-year follow-up data from the ULTIMATE RCT showing a significant reduction of target vessel failure with IVUS-guided PCI in the bifurcation subgroup (HR 0.48; 95% CI [0.27–0.87]).73 These data matched the findings in the overall trial population.
The 5-year results from DK CRUSH-II identified a significantly reduced rate of MI of 1.8% in the IVUS-guided subgroup compared with 5.4% in the angiographic-guided subgroup for non-LM bifurcation lesions (p=0.043).74
The long-term effect of IVUS-guided non-LM bifurcation treatment was first assessed in 2010 by Kim et al.75 IVUS-guided bifurcation stenting significantly reduced 4-year all-cause mortality compared to angiographic guided stenting only (HR 0.31; 95% CI [0.13–0.74]; p=0.008).
In a propensity score matching analysis by Kim et al., 974 patients with non-LM bifurcation lesions who underwent DES implantation with or without IVUS-guided PCI were analyzed.76 The IVUS-guided strategy was associated with larger post-stent lumen diameters in the MV and SB compared to the angiographic-guided group. Notably, the incidence of death or MI was significantly lower in the IVUS-guided group compared with the angiography-guided group (3.8% versus 7.8%; HR 0.44; 95% CI [0.12–0.96]; p=0.04).
A 2020 meta-analysis that included five studies and 7,830 patients with CBLs, demonstrated that the incidence of MACE in the IVUS-guided group was lower than those in the angiography-guided group (OR 0.55; 95% CI [0.42–0.70]; p<0.0001).77
Data on Optical Coherence Tomography-guided Non-left Main Bifurcation Stenting
Retrospective data and several case reports have provided further insights into the feasibility of online visualization of the guidewire position in CBLs (especially for distal cell wire crossing) using OCT.78,79
The OPTIMUM trial randomized 110 patients to either 3D optical frequency domain imaging (3D-OFDI)-guided PCI or angiography-guided PCI.80 After MV stenting and POT, those randomized to the 3D-OFDI-guided strategy underwent 3D-OFDI-guided rewiring into the jailed SB. In contrast, patients randomized to the angiography-guided strategy underwent rewiring using conventional angiography/fluoroscopy. The results showed that the rate of incomplete stent apposition was significantly lower with the 3D-OFDI-guided strategy than with the angiography-guided strategy (19.5% versus 27.5%; p=0.008). The study implies that 3D-OFDI-guided PCI significantly reduces the rate of overhanging struts at the SB ostium, which seems to be an essential risk factor for neointimal growth for restenosis of the SB-ostium and stent thrombosis.81
Conclusion of Intravascular Imaging Non-left Main Bifurcation Stenting
IVUS and OCT have significantly improved the evaluation of CBLs in interventional cardiology over many years. Notably, no RCT has been published specifically for bifurcation imaging-guided PCI. Registry data, subgroup analyses, and observational data on IVUS and OCT provide significant insights for pre- and postprocedural decision-making and reduce the incidence of MACE. For this reason, the European Association of Percutaneous Cardiovascular Interventions expert consensus on the clinical use of intracoronary imaging recommends assessing the vessel wall morphology thoroughly, the degree of calcification, and lesion characteristics before stent implantation and the use of intracoronary imaging for postprocedural control and correction of the achieved results for PCI.82,83
The ongoing DK CRUSH VIII trial will shed light on the superiority of IVUS-guided versus angiography-guided DK crush-stenting in patients with CBLs.84 Similarly, the results of the OCTOBER trial will elucidate the role of OCT-guided PCI in patients with CBLs.85 If these trials provide convincing evidence regarding the benefit of IVUS and OCT on the outcome of stent optimization in patients with CBLs, the relevant data may serve as an impetus for changing clinical practice and upgrading IVUS and OCT to a class 1 recommendation in guidelines.
Conclusion
Finding the optimal stenting strategy for CBLs is still one of the most debatable topics among invasive cardiologists. The current evidence suggests that a PS approach, which can be upgraded to a two-stent strategy (T/TAP or culotte) in case of SB loss, should be the primary approach for most cases. Nevertheless, one should recognize true complex CBLs where an upfront two-stent strategy (inverted T/TAP, inverted culotte, DK crush) should be adopted by securing (stenting) the SB first. Despite scarce randomized data on CBLs, intracoronary imaging and debulking devices should be implemented into daily practice to achieve better short- and long-term results.