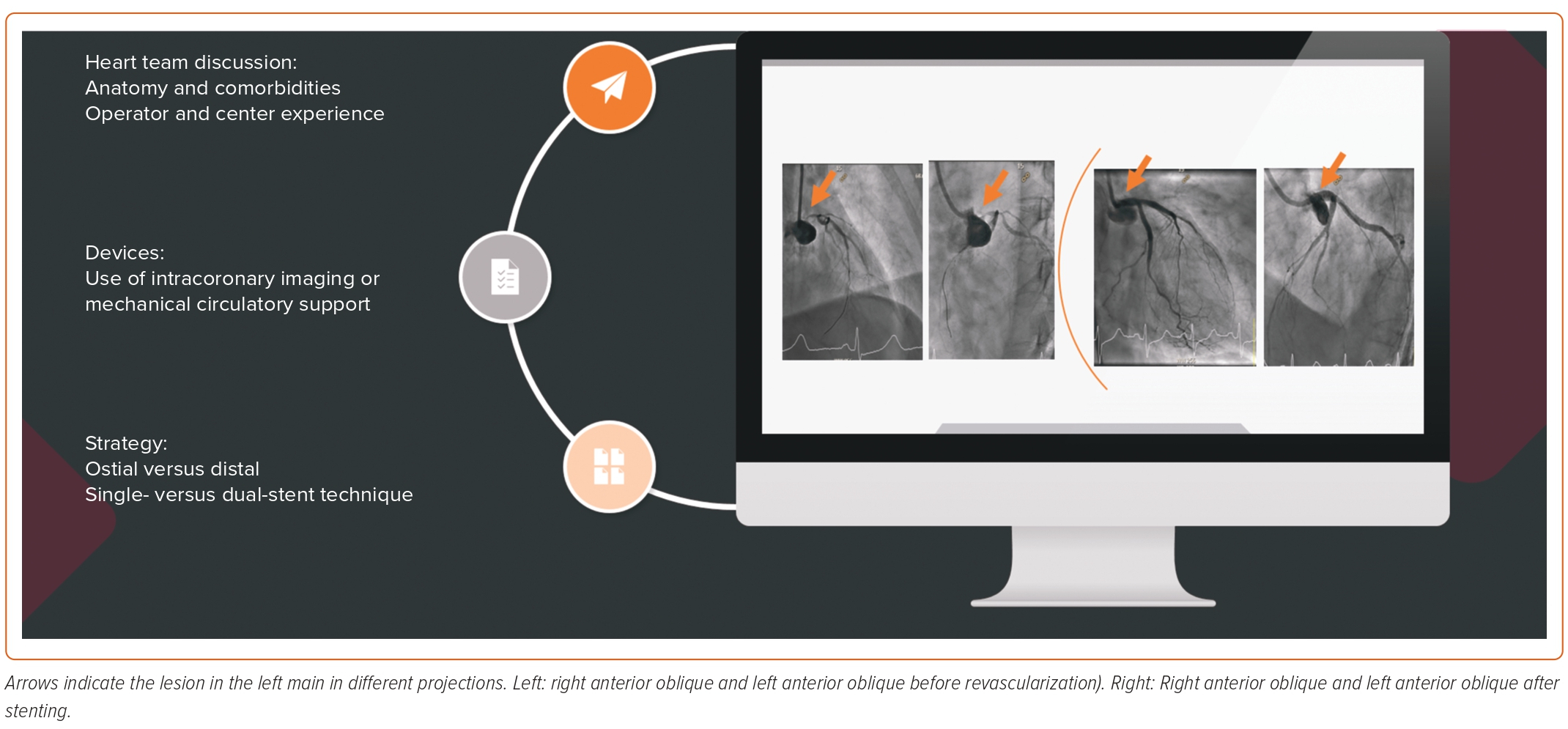
Given the rapid developments in drug-eluting stent platforms and the adoption of bifurcation techniques, percutaneous coronary intervention (PCI) of left main (LM) disease has become a common procedure in modern day catheterization laboratories.1,2 In fact, percutaneous revascularization of patients with LM disease and a low SYNTAX score has been recommended in the most recent American College of Cardiology and European Society of Cardiology clinical practice guidelines.3,4 However, it is critical to understand the evidence for the new recommendation and define the optimal stenting technique for LM disease. This review provides a critical appraisal of the relevant trials, summarizes different stenting techniques for distal LM disease, and illustrates the role of intracoronary imaging and mechanical circulatory support devices (Figure 1).
Contemporary Evidence
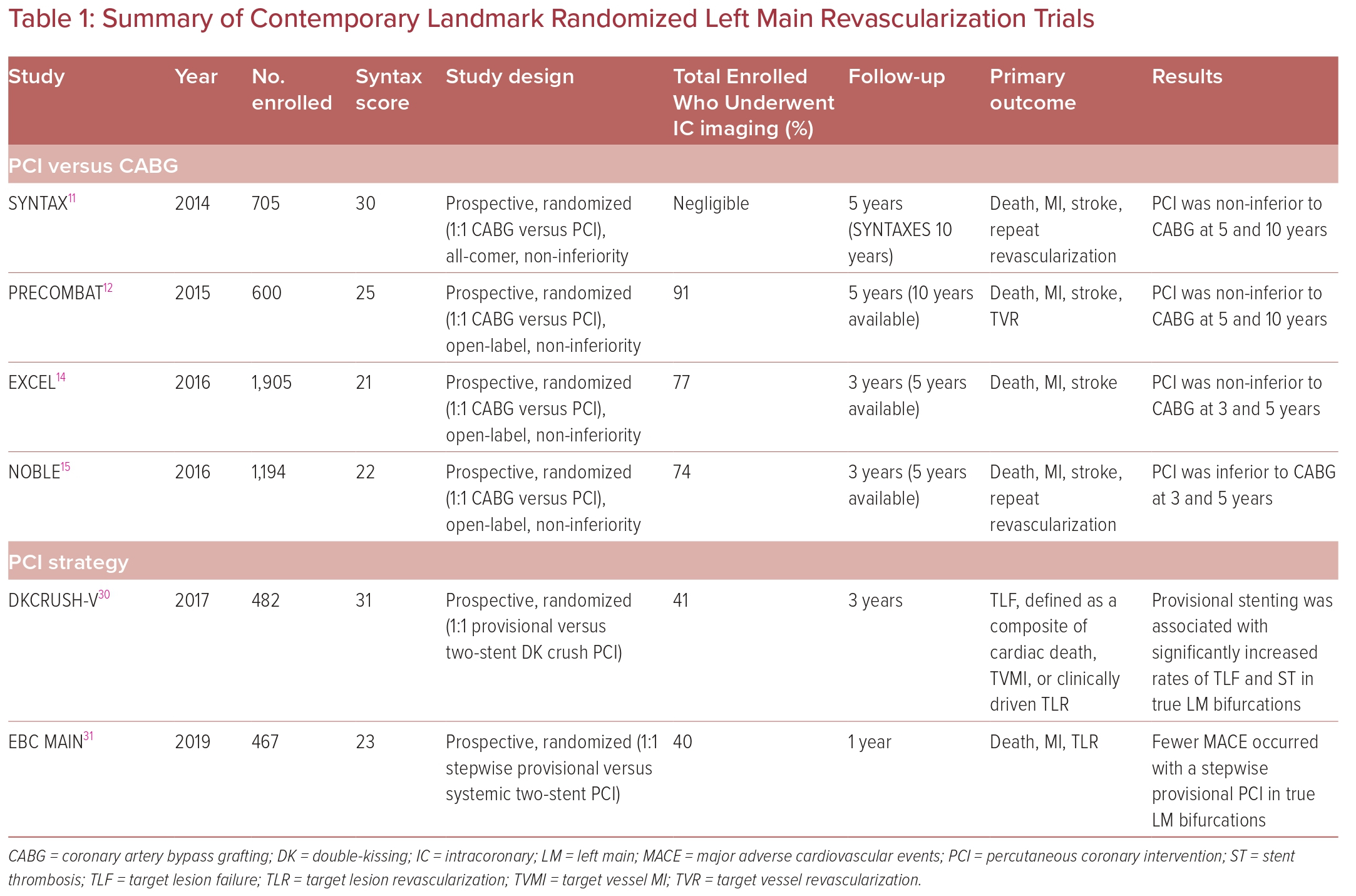
Since Andreas Gruntzig first reported his successful percutaneous balloon angioplasty of two left main arteries in 1979, the debate on the appropriateness and effectiveness of this intervention have been studied and compared to the standard-of-care coronary artery bypass grafting (CABG) in many randomized controlled trials (RCTs).5 Despite more than 20 years of marked technological advances in stent technology and bifurcation strategies, the debate continues. Table 1 provides a summary of the most notable trials of LM revascularization.
One of the first retrospective analyses comparing PCI and CABG to medical therapy in patients with multivessel coronary artery disease (CAD) was the APPROACH trial, published in 2001.6 The APPROACH trial demonstrated the superiority of revascularization over medical therapy alone. The 5-year survival was 91.4% with CABG, 91.9% with PCI, and 82.9% with medical therapy (p<0.001).6 However, in patients with severe LM disease, the benefit of CABG exceeded that of PCI (HR 0.30; 95% CI [0.17–054]).6
Subsequently, several RCTs confirming the feasibility and safety of PCI for LM disease were published.7,8 These RCTs provided a direct head-to-head comparison of both therapies and led to a change in the guidelines for PCI of LM disease. Erglis et al. conducted the first head-to-head comparison of bare metal stents (BMS) with the newer drug-eluting stents (DES) in patients undergoing revascularization for unprotected LM coronary artery (ULMCA) disease and demonstrated the superiority of DES compared with BMS.9 Of note, all interventions in that trial used intravascular ultrasound (IVUS).
One of the first RCTs comparing ULMCA stenting to CABG reported a significant increase in left ventricular (LV) ejection fraction at the 12-month follow-up after PCI than after CABG (mean ± SD 3.3 ± 6.7% versus 0.5 ± 0.8%, respectively; p=0.047).10 PCI was associated with a lower 30-day major adverse cardiovascular and cerebrovascular events (MACCE; p=0.03), as well as a shorter hospital stay (p=0.0007). There was no difference in survival at 2 years.10
The more robust data supporting ULMCA PCI were generated by a subgroup analysis out of the SYNTAX trial comparing the use of first-generation DES with CABG in LM disease.11 This was the largest trial, including 705 patients with ULMCA disease. The analysis itself was both predetermined and well powered. MACCE at 5 years was 36.9% following PCI and 31.0% following CABG (HR 1.23, 95% CI [0.95–1.59]; p=0.12). The mortality rate at 5 years was 12.8% for PCI and 14.6% for CABG (HR 0.88, 95% CI [0.58–1.32]; p=0.53). Of concern was the significantly higher rate of stroke following CABG than PCI (4.3% versus 1.5%, respectively; HR 0.33, 95% CI [0.12–0.92]; p=0.03).11 Unsurprisingly, the rate of repeat revascularization was higher after PCI (26.7% versus 15.5; HR 1.82, 95% CI [1.28–2.57]; p<0.01).11 In that study, MACCE rates were similar in those with low or intermediate SYNTAX scores; however, MACCE rates were significantly higher following PCI in those with a high score (>33).11 The results of that trial shaped the current European guidelines that deem PCI an appropriate alternative to CABG in ULMCA disease with low to intermediate SYNTAX score, with an upgraded level of evidence A.4 However, the US guidelines did not change until 2021 after the publication of the EXCEL and NOBLE trials, which are discussed later. The US guidelines afforded a class 2a indication (level of evidence: B non-randomized) to PCI for patients in whom equivalent revascularization can be achieved with both PCI and CABG.3
The PRECOMBAT trial was a South Korean trial published in 2015.12 That trial noted no significant difference between patients undergoing PCI using a sirolimus-eluting stent and patients undergoing CABG at the 5-year follow-up.12 The 10-year results reconfirmed these findings, whereby the primary endpoint (the incidence of MACCE, defined as a composite of mortality from any cause, MI, stroke, or ischemia-driven target vessel revascularization [TVR]) occurred in 29.8% of the PCI group and in 24.7% of the CABG group.13 The 10-year rate of the composite of death, MI, and stroke in the PCI and CABG groups was 18.2% and 17.5%, respectively (HR 1.00, 95% CI [0.70–1.44]), and all-cause mortality was 14.5% and 13.8% (HR 1.13, 95% CI [0.75–1.70]), respectively.13 Once again, ischemia-driven TVR was higher in the PCI than CABG group (16.1% versus 8.0%; HR 1.98, 95% CI [1.21–3.21]).13 The value of the PRECOMBAT trial is in the long-term, 10-year results, which have not been previously available for LM PCI trials.
As noted earlier, there are two contemporary landmark trials assessing the outcomes of second-generation DES in LM PCI versus CABG in the recent era. The EXCEL trial enrolled 1,905 patients with a low–intermediate SYNTAX score of <33 who were randomized to PCI or CABG.14 The primary endpoint, a composite of all-cause death MI, and death in 3 years, occurred in 14.7% of patients in the CABG group and in 15.4% of patients in the PCI group (p=0.018 for non-inferiority; p=0.98 for superiority).14 The secondary outcome of death, stroke, or MI at 30 days occurred in 4.9% of patients in the PCI group and in 7.9% of patients in the CABG group (p<0.001 for non-inferiority; p=0.008 for superiority).14 At 3 years, the reported primary outcome was 23.1% for PCI compared with 19.1% for CABG (p=0.01 for non-inferiority; p=0.10 for superiority), indicating a lower event rate in the PCI group at 30 days which converged by 3 years.14 The NOBLE trial enrolled 1,201 patients with a low–intermediate SYNTAX score who were randomized to PCI or CABG.15
The outcomes of the NOBLE trial were similar to those of EXCEL at 30 days, but at 5 years PCI was inferior to CABG in the NOBLE trial owing to a higher rate of repeat revascularization. It is important to note that EXCEL excluded repeat revascularization in the primary outcome. Furthermore, NOBLE primarily used biolimus DES and first-generation DES were used in 10% of the cohort, whereas in EXCEL only second-generation sirolimus-eluting stents were used. The contradicting results of these two trials prompt an individualized heart team approach for each patient. The lower repeat revascularization rates with CABG warrant transparent discussions, particularly because repeat revascularization was found to be an independent predictor of death, stroke, and MI in the SYNTAX trial.11
Another important observational cohort study was the MAIN-COMPARE registry, which assessed the 10-year outcomes of LM PCI versus CABG.16 No significant difference was detected between both groups in the adjusted risk of death and composite outcomes. The trial also reported a higher rate of TVR in the PCI group.16 Moor et al. conducted a meta-analysis that included the four major trials (SYNTAX, PRECOMBAT, EXCEL and NOBLE).17 That analysis revealed a higher incidence of MACCE at 3–5 years with PCI than CABG (23.3% versus 18.2%; OR 1.37, 95% CI [1.18–1.58]; p≤0.0001); this was once again primarily driven by repeat revascularization, with no statistically significant differences in mortality, MI, or stroke.17
As such, the 2021 American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions guideline for coronary revascularization ascribed a class 2a indication (level of evidence: B non-randomized) to PCI for patients in whom equivalent revascularization can be achieved with both PCI and CABG. This recommendation does not include anatomic or clinical risk profile.3 The European Society of Cardiology’s guidelines in 2018 assigned a class 1 and 2a, or 3 recommendation based on the SYNTAX score tertile.4
Stenting Techniques
Stenting techniques depend largely on LM anatomy, in particular the presence of a distal bifurcation or ostial disease. These require meticulous planning, positioning, and optimization of the revascularization technique. It is critical that operators understand the caveats and anticipate complications. As indicated by the previously discussed randomized trials, operator experience is key for the success of these complex LM interventions.
Aorto-ostial Left Main Percutaneous Coronary Intervention Techniques
Traditionally, ostial lesions are defined as those occurring within 3 mm of the origin of the vessel. Aorto-ostial lesions have variable take offs from the aorta due to the three-dimensional morphology and limited visualization on two-dimensional fluoroscopy. Because of the increased elasticity and muscular layer of the aorta, these lesions can be resistant to dilatation and prone to recoil. Optimal aorto-ostial stent deployment requires the coverage of the whole aorto-ostial landing zone (AOLZ) with the proximal stent struts. The AOLZ is defined as the area located within 1 mm of the aorto-ostial plane.18 There have been many techniques and devices developed over the years to avoid a geographical miss and to optimize and standardize aorto-ostial stenting. For ostial lesions, it is critical to select a non-aggressive guide to avoid deep intubation of the vessel that provides adequate support.
The more conventional technique is angiographically assisted placement under fluoroscopy alone. This technique relies on the fluoroscopic two-dimensional image often in a single projection, which is the least accurate of the adopted techniques. Rubinshtein et al. reported a rate of 87% for a geographical miss in aorto-ostial stent implantation using high-resolution coronary CT angiography. This confirms the poor accuracy and reproducibility of fluoroscopically implanted LM stents.18 The aorta free-floating wire technique is another strategy often used for ostial LM stenting. Two wires are required, whereby one wire is placed in the designated vessel and the other is free floating along the aortic wall. The free-floating wire placed a few millimeters away from the engaged guide catheter, the aortic wall, and the coronary ostium is used as a marker to facilitate positioning. The relationship between the wire and the stent may be variable depending on the fluoroscopic views. The tail-wire technique, also known as Szabo’s technique, was first described in 2005.19 Here, the operator places a ‘stop wire’ across the proximal strut of the stent, which could facilitate fixing the proximal stent at the exact ostium of the target vessel.19 In 2012, clinical experience and bench top testing confirmed significant asymmetry and deformation of the proximal wire stent cell using this technique.20 Flaring the ostium can be achieved using either a larger post-dilation balloon at the ostium or a dedicated flaring system, such as the Flash Ostial system (Ostial Corporation). This device is comprised of two balloons, a high-pressure, coronary-size balloon and a larger, low-pressure, anchoring balloon.21
Intracoronary imaging is beneficial both before and after stenting. This has been recommended by expert operators and the 16th European Bifurcation Club consensus document for LM interventions.22,23
Distal Left Main Disease Percutaneous Coronary Intervention Techniques
Selecting the most appropriate distal LM bifurcation PCI strategy, either provisional single stenting or an a priori two-stent strategy, remains debatable. Owing to advances in stent platforms and image-guided PCI, operators can more confidently select the stenting approach and further optimize their results.2,24,25 Current clinical guidelines emphasize the role of the heart team in identifying the most appropriate revascularization option based on clinical and anatomical factors, as well as center and operator expertise.3 LM disease involving the distal bifurcation requires special considerations. Regardless of the technique, LM bifurcation lesions should be approached systematically by evaluating the significance of the side branch (SB) lesion and its risk of being compromised based on known factors. The DEFINITION II trial identified the most widely accepted criteria for significant SB disease, namely an SB ≤10 mm in length and ≤2.5 mm in diameter.26 One major and two minor criteria are required to further determine a stepwise provisional or upfront two-stent strategy. The major criteria are a lesion that is ≥10 mm in length and SB stenosis ≥70%; the minor criteria include a bifurcation angle <45° or >70°, a main vessel that is <2.5 mm in diameter, a lesion that is >25 mm in length, and the presence of thrombus, chronic total occlusion, or calcification.26
Based on the above, a stepwise provisional single-stent technique is the most commonly adopted LM PCI strategy. A stent is implanted in the main vessel (with the stent size selected according to the distal reference diameter of the main vessel) and optimal post-dilation of the proximal stent segment (in the LM) with an appropriately sized non-compliant balloon to achieve optimal strut apposition and expansion, often under the guidance of intracoronary imaging.27
Lee et al. assessed the prognostic value of fractional flow reserve (FFR) in a jailed left circumflex artery (LCx) following simple crossover stenting of LM lesions.28 That study demonstrated a marked discordance between angiographic and FFR findings after stenting. An FFR ≤0.80 was an independent predictor of ostial LCx failure after 5 years. The investigators concluded that FFR measurement in a jailed LCx after PCI of the LM using a crossover approach can be useful in determining the most suitable treatment strategy and may simplify procedures.28
A final kissing inflation to open the struts into the LCx remains controversial. There is no evidence for a reduction in target lesion failure (TLF) or TVR with kissing inflation in a provisional strategy. However, it may, in theory, facilitate future access without penalty of increased events.
The DEFINITION trial revealed that a two-stent strategy with a double-kissing (DK) crush technique was superior to provisional stenting for complex LM bifurcation lesions.29 In the DEFINITION trial, 653 patients were randomized into two parallel groups. In the two-stent group, an SB stent was not implanted in 7.9% of cases. A DK crush was performed in 78% of cases and a culotte was performed in 18%.29 In those fulfilling the DEFINITION II criteria, a systematic two-stent strategy was associated with a reduction in TLF (6.1% in the two-stent group versus 11.4% in the provisional stent group; p=0.019) at the 1-year follow-up.26
More recent RCTs comparing bifurcation strategies for distal LM have been published, namely the EBC-MAIN and DKCRUSH-V trials, which compared provisional stenting to the two-stent strategy.30,31 In the EBC-MAIN trial, the study population was older (71.1 versus 64.5 years), the operators had less experience (≥150 versus ≥300 PCI/year), and the SYNTAX score was lower (23 versus 31) with a shorter SB lesion length (7 versus 16 mm).30,31 This could explain the lower conversion rate to a two-stent technique with the provisional strategy in the EBC-MAIN than DKCRUSH-V trial. In terms of outcomes, the DKCRUSH-V trial reported a lower rate of the composite endpoint of cardiac death, target vessel MI, or clinically driven TLR, whereas the EBC-MAIN trial showed numerically fewer major adverse cardiovascular events (MACE) in a stepwise layered provisional approach, although this did not reach statistical significance. Geographic differences in the studied populations or differences in operator expertise with the given techniques cannot be dismissed. Figure 2 is an example of a T and small protrusion (TAP) technique using enhanced fluoroscopic imaging.
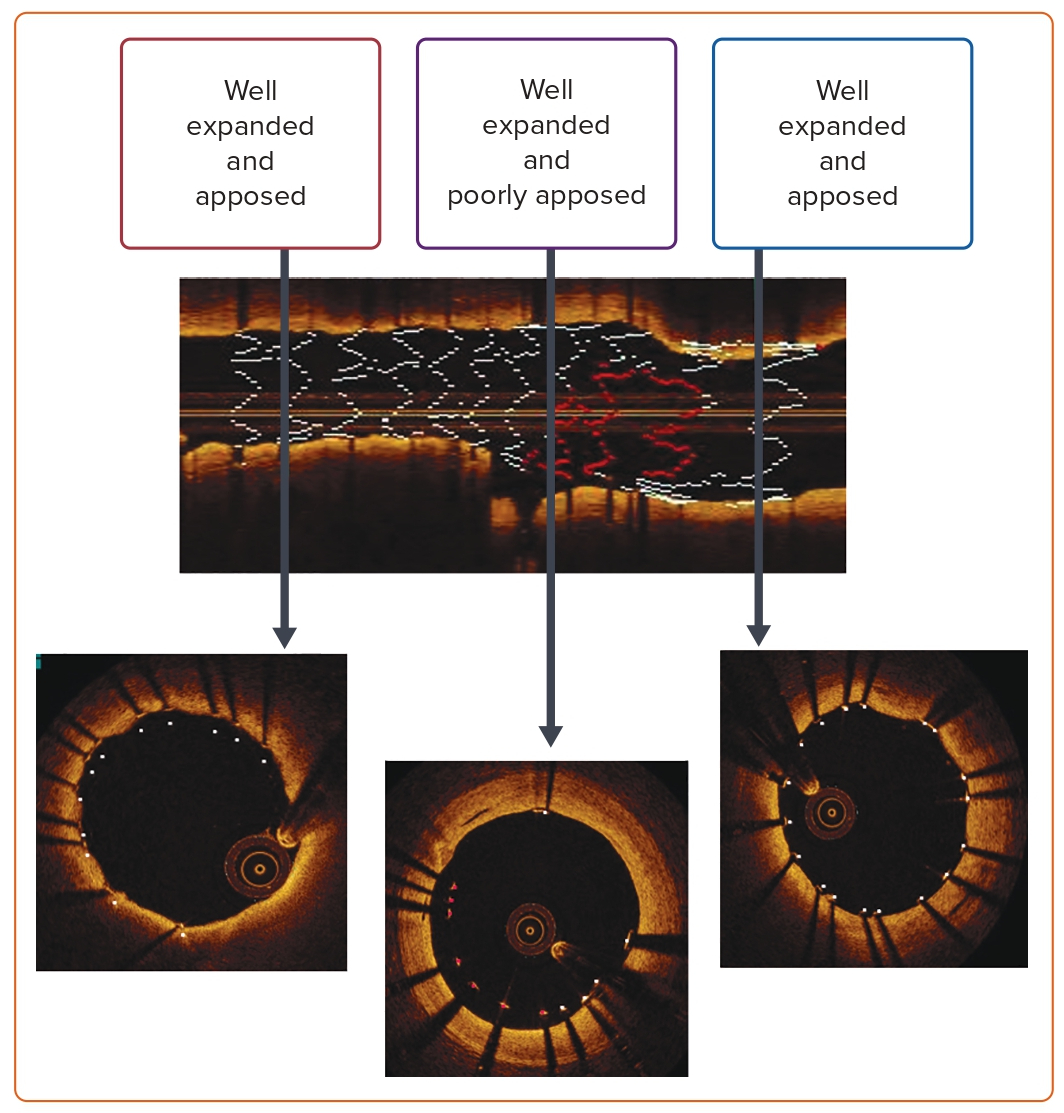
Intracoronary Imaging to Guide Left Main Percutaneous Coronary Intervention
Fluoroscopy is the initial diagnostic step for LM disease. However, with advances in imaging technologies, IVUS and optical coherence tomography (OCT) have become adjunctive tools detailing the morphology of the LM lesion, severity of disease, plaque extension, ostial disease of daughter branches, and bifurcation anatomy. As such, imaging helps with the selection of the revascularization strategy (including the need for plaque modification and an upfront two-stent strategy) and improves outcomes following LM PCI.24,32
Intravascular Ultrasound
IVUS-guided PCI is associated with a significant reduction in death, MACE, and stent thrombosis compared with angiography alone.34 A meta-analysis of a single randomized trial and 10 observational studies involving 19,619 patients confirmed the utility of IVUS.33 IVUS provides a tomographic 360° scan of the vessel that measures the minimum lumen area (MLA), permitting objective quantification of the stenosis. Although an MLA of 6 mm2 is considered to be a valid cut-off for management decision, there are population differences reported in a South Korean study emphasizing an MLA cut-off of 4.5 mm2.35 However, it is widely accepted that a significant stenosis by IVUS is defined as an MLA of <5 mm2. There is currently no consensus on the MLA threshold for intermediate lesions. An MLA value of 6 mm2 was extracted from Murray’s law where an MLA of 4 mm2 is considered the ischemic threshold of the branches and has been derived from FFR trials.36,37
IVUS has some challenges in the case of a short LM length, eccentric lesions, diffuse CAD, calcification, overlapping vessels, and catheter-induced spasm.35 Therefore, precise assessment may entail additional evaluation by either imaging with OCT or, more commonly, a physiological study using FFR or both, each with its own limitations. An FFR cut-off of <0.08 indicates a significant flow-limiting stenosis that usually correlates with IVUS, as shown by Kang et al.38 The current consensus document recommends the use of IVUS in LM PCI for stent optimization both before and after implantation, assessment of the mechanism of stent failure, and the evaluation of an indeterminate LM lesion, as in the LITRO study.39,40 In that study, revascularization of the LM was performed in 152 of 168 patients with an MLA <6 mm2 and was deferred in 179 of 186 patients with an MLA of ≥6 mm2. Significant scatter was observed between the groups using angiographic parameters. At the 2-year follow-up, cardiac death-free survival was 97.7% in the deferred group, compared with 94.5% in the revascularized group (p=0.5).40 The event-free survival was 87.3% and 80.6% in the deferred and revascularized groups, respectively (p=0.3). Only 4.4% of enrolled patients in the deferred group required subsequent revascularization.40
Optical Coherence Tomography
Several studies have demonstrated the non-inferiority of OCT compared with IVUS for image-guided PCI.41–43 Despite its higher resolution, there is paucity of data regarding OCT-guided LM intervention owing to its limited utility in ostial lesions and short LM arteries. These are often the cases when it becomes difficult to clear blood from the LM to avoid artifacts. Unfortunately, most trials conducted on OCT-guided PCI did not include LM interventions, particularly in ostial lesions. In contrast, OCT-guided PCI of the mid-shaft and distal LM bifurcation has attained both feasibility and safety.44,45 Despite accurate, reproducible, and quantitative coronary dimension measurement by OCT, there is no consensus on a cut-off point beyond which deferring LM PCI is deemed safe. Figure 3 illustrates the accuracy of OCT in determining the appropriate size and length of the disease. OCT-guided PCI of the LM has not been incorporated in international guidelines to date.3,4
Mechanical Circulatory Support
Mechanical circulatory support (MCS) is often considered for LM PCI in the setting of both shock and high-risk procedures. To date, there is no consensus on the definition of high-risk PCI. For most operators, high-risk PCI generally encompasses those with impaired LV systolic function, complex coronary anatomy, and comorbidities. These are generally the patients who are deemed inoperable or too high risk for surgical revascularization. Several devices have been used in such settings, including the catheter-based miniaturized ventricular assist axial device (Impella), TandemHeart, venoarterial extracorporeal membrane oxygenation (VA-ECMO), and intra-aortic balloon pump (IABP).
Briefly, the Impella accounts for approximately 31.9% of all MCS devices.46 Impella CP (Abiomed) is the most frequently used in the current era and provides blood flow up to 4 l/min, thereby unloading the LV throughout the cardiac cycle and increasing end organ perfusion through a 14-Fr femoral arterial system.47 Data from observational studies and registries demonstrate that Impella-assisted high-risk PCI is safe and effective, with a low rate of periprocedural complications. A large multicenter retrospective registry from Europe (Europella) reported successful Impella hemodynamic support for high-risk PCI, of which 53% were for LM interventions.48 At 30 days, the mortality rate was 5.5% and the rate of vascular complications was 4%.48 Similar results were reported from the USpella Registry, which was a single-center retrospective evaluation of the efficacy and safety of the Impella device for unprotected LM PCI in patients with LV systolic dysfunction.49 In that study, the 30-day mortality rate was 2.36%.49
TandemHeart (TandemLife, LivaNova) is a 21 Fr drainage catheter that is inserted into the left atrium (LA) using a trans-septal approach from the femoral vein. The LA blood is aspirated by the centrifugal pump and propelled into the arterial circulation through a 15–17 Fr return catheter. This device provides circulatory support up to 5 l/min. It permits reduction of the LV preload, filling pressures, workload, and myocardial oxygen demand, and provides adequate end organ perfusion. Unlike Impella, TandemHeart can be used in the presence of a pre-existing LV thrombus and aortic stenosis. However, the system’s performance is limited in the presence of right ventricular failure, pulseless ventricular arrhythmias, and asystole. Vranckx et al. reported a single-center experience of the TandemHeart system where safety and efficacy were evaluated during the treatment of unprotected LM coronary artery.50 The authors reported an 89% survival rate at 6 months, with a rate of vascular access site complications of 44.4%.50 Another retrospective cross-sectional analysis of prospectively collected data from the Mayo Clinic demonstrated a high success rate when TandemHeart was used for high-risk PCI (LM and multivessel PCI was performed in 62% of patients).51 The procedural success rate was 97% and 6-month survival was 87%, whereas major vascular complications occurred in 13% of cases.51
VA-ECMO provides both heart and lung support to a maximum blood flow up to 7 l/min. It reduces LV preload, reduces myocardial oxygen consumption, improves coronary perfusion, provides oxygenated blood to end organs, and improves mean arterial pressure, but increases LV afterload. A retrospective study performed in VA-ECMO-assisted high-risk PCI (in which 78% of patients had LM coronary artery disease), the survival rate was 93% and the rate of vascular access site complications was 14%.52 In another retrospective single-center registry of patients with high-risk PCI receiving VA-ECMO support in which 78.6% of enrolled patients had LM stenosis, successful revascularization was achieved in 92.8%, with a MACE rate of 28% during the hospital stay.53
Finally, IABP is one of the frequently used devices given its availability, cost, and ease of insertion with a lower profile sheath (7–8 Fr). BCIS-1 was the first RCT of IABP in elective high-risk PCI.54 There was no significant difference in in-hospital MACE rates in those with and without IABP; however, the long-term all-cause mortality at 51 months was significantly less in the elective IABP group (HR 0.66, 95% CI [0.44–0.98]; p=0.039).54 Mishra et al. demonstrated in their multivariate analysis that prophylactic use of IABP is an independent predictor of survival at 6 months.55 The authors noted that vascular complications were low and comparable between the high-risk patients who received a prophylactic IABP and those who required rescue IABP only after intraprocedural complications. However, the incidence of major bleeding was significantly higher in the rescue IABP group (15% vs 3%; p=0.03).55 Overall, careful selection of patients and anatomy that warrant MCS needs to be weighed against the potential risks. Operator and center experience likely affect outcomes.
Conclusion
LM revascularization warrants particular attention given the large territory subtended. Current guidelines recommend PCI for patients with a low SYNTAX score and emphasize the role of the heart team in determining the most appropriate revascularization strategy for patients, bearing in mind operator experience, patient comorbidities, and lesion complexity.39 Although evidence suggests better outcomes with image-guided PCI, the guidelines are yet to acknowledge its utility. The totality of evidence suggests a stepwise provisional strategy is safe. The review alludes to the geographic differences in studies, and larger randomized studies exploring genetic and geographic differences would be useful in guiding practice.