Spontaneous coronary artery dissection (SCAD) is a cause of acute coronary syndrome (ACS) secondary to an epicardial coronary artery dissection that is not associated with atherosclerosis or trauma and is not iatrogenic.1 Myocardial injury is caused by the development of a dissection flap or intramural hematoma in the tunica media leading to coronary obstruction. Two hypotheses have been proposed to explain the pathophysiological process: the ‘inside-out’ hypothesis, in which blood enters the subintimal space after an endothelial–intimal disruption, and the ‘outside-in’ hypothesis, in which intramural hematoma arises de novo by rupture of the vasa vasorum.1
Optical coherence tomography (OCT) provides high-resolution images of the coronary vasculature, which sheds further light on both pathophysiological processes. A study of 68 vessels in 65 patients with acute SCAD and OCT imaging supported both the inside-out and the outside-in hypotheses. Presence of fenestration or communication between the true and false lumen was noted in 25 vessels, consistent with the inside-out hypothesis. In 43 vessels there was an absence of fenestration, leading to obstruction by expansion of the false lumen and decreased area for blood flow through the true lumen, consistent with the outside-in hypothesis.2 The study showed no differences in the vasa vasorum density in SCAD patients and controls, suggesting a potential alternate mechanism for the outside-in hypothesis.2
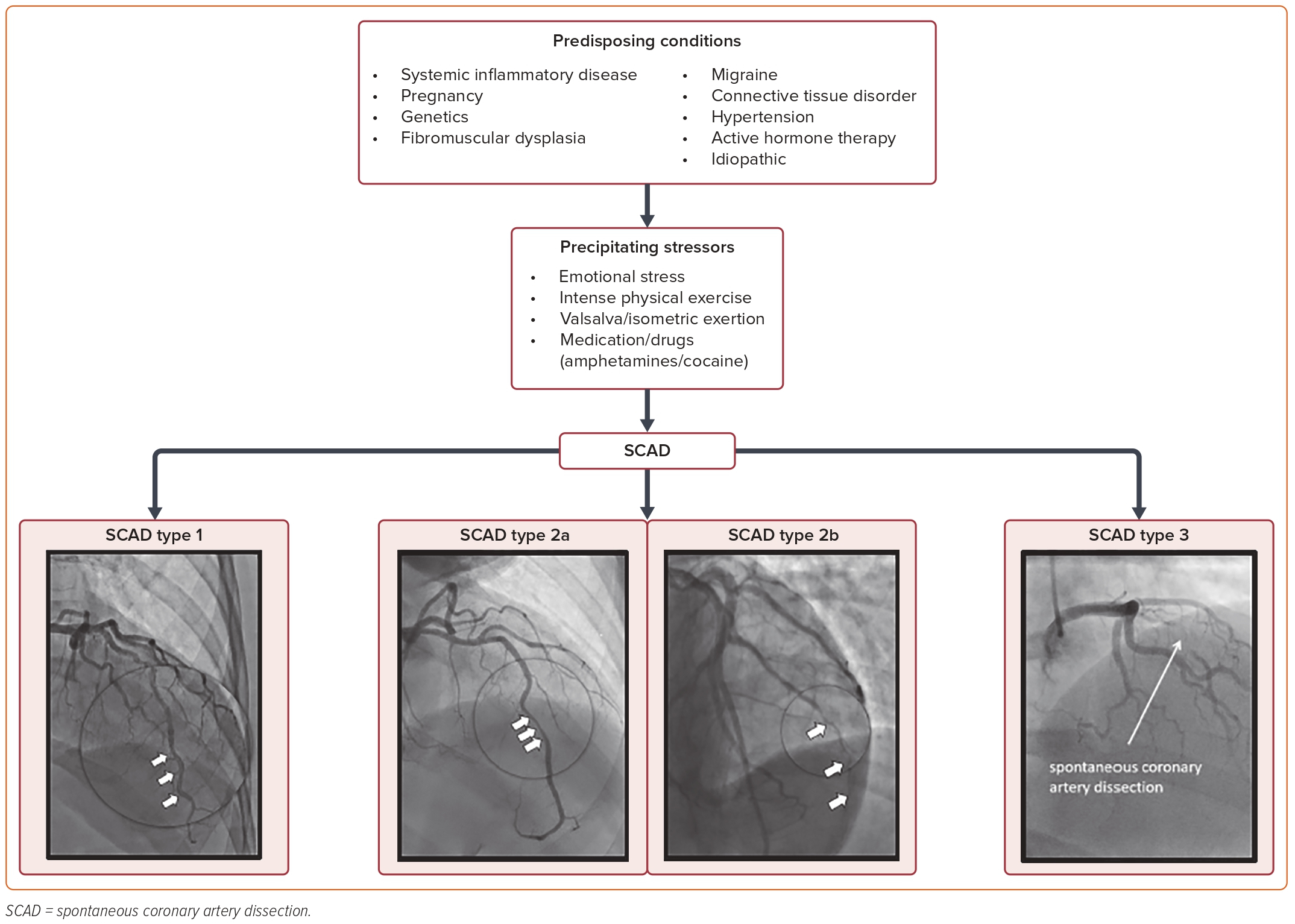
Early invasive coronary angiography is the gold standard for the diagnosis of SCAD.1 SCAD can be divided into four subtypes based on angiographic appearance, three of which are outlined in Figure 1. Type 1 shows contrast staining delineating a flap or double lumen and can be seen in up to 48% of cases.1,3 Type 2 is most common and is seen in up to 67% of cases, which are further divided into type 2a, characterized by a long tubular stenosis and subsequent restoration of a normal caliber vessel distally, and type 2b, which involves diffuse narrowing that extends to the distal tip of the vessel.1,3 Type 3 is a focal stenosis that may mimic atherosclerotic disease and is seen in up to 3.9% of cases, and type 4 is the least common: total occlusion of a distal vessel often only confirmed during coronary intervention.1,3,4 Other angiographic findings of relevance include increased coronary tortuosity, reduced or absent atherosclerotic disease, and association of dissection sites with myocardial bridging.3 This angiographic classification has improved the recognition and accuracy of SCAD diagnosis and increased scientific interest.5,6
In the past decade, our understanding of SCAD has shifted from ‘rare,’ to multicenter studies collaborating to increase awareness and improve patient care. Much of the SCAD literature has originated in the last 10 years (72%), with the last 5 years accounting for 80%.7 Despite an increased awareness among patients and providers, SCAD continues to be underdiagnosed or misdiagnosed, and is usually mistaken for atherosclerotic coronary disease. An emphasis on recognition and diagnosis of SCAD is paramount to providing appropriate care and avoiding the potential harm from inappropriate treatment and procedures.
Epidemiology and Demographics
SCAD has been described in individuals ranging in age from 18 to 84 years, largely debunking the notion that it is primarily a disease of the young.3 It has a unique demographic and risk factor profile, with women comprising approximately 90% of cases.8 SCAD is believed to be the etiology of MI in 24–35% of women below the age of 60 years.9 The mean age of patients with SCAD ranges from 44 to 53 years old.3 Recent analysis of the Canadian SCAD cohort suggests that men with SCAD tend to be younger, with a mean age of 49.4 ± 9.6 years in men versus 52.0 ± 10.6 years in women.10
For the purposes of this article, we have adopted the SAGER guidelines for sex and gender reporting. When addressing sex-specific biological attributes (including discussions of chromosomes, sex organs, and endogenous hormonal profiles), we will designate sex differences with the terms "female" and "male.” In contrast, when discussing societal impact factors or in studies in which gender was self-reported, we will designate gender differences with the terms "women" and "men." We acknowledge there are data limitations in this review as many historic studies did not ask participants to specify biological sex versus self-designated gender.
A study using data from the National Readmission Database assessed adult hospitalizations involving a primary diagnosis of SCAD and found that men were more likely than women to have atherosclerotic risk factors, including prior history of diabetes, hypertension, hyperlipidemia, and chronic kidney disease.11 Other studies suggest that cardiovascular risk profiles are similar between men and women with SCAD.10 Additionally, atherosclerotic risk factors appeared to be similar in SCAD and non-SCAD patients who presented with ACS, despite the absence of atherosclerosis commonly observed through angiography in SCAD patients.12 The cause of SCAD is multifactorial with genetic factors, hormones, and arteriopathies playing a role, as well as environmental or emotional stressors precipitating the event. Figure 1 outlines the predisposing conditions, precipitating factors, and angiographic classification of SCAD. Evidence suggests that SCAD is preceded by an emotional event in 40–56% of cases, while 18–24% of cases are preceded by heavy physical exertion.13 McAlister et al. suggest that men are more likely to experience physical triggers and women are more likely to have emotional triggers.10 Migraine headaches (40%), depression (25%), and anxiety (15%) are frequently associated symptoms.14
When comparing patient profiles of men and women in SCAD events, men had a lower risk of prior MI than women (0.8% versus 7.0%) and were less likely to have a history of depression (9.8% versus 20.2%) and migraines (17.9% versus 35.8%).9,10 In that same study, the absolute number of men with anxiety was also less but was ultimately not significant.9,10 Men and women had similar levels of moderate stress, but women were significantly more likely to have high perceived stress (3.5% versus 11.0%).15 Other SCAD registries also report higher rates of self-reported anxiety and depression in women than in men.16 There is a school of thought that depression may be a bystander in SCAD as opposed to a specific risk factor, given the higher prevalence in women in the general population.15 However, studies focused on coronary syndrome in women corroborate the idea that women with comorbid depression have a higher incidence of cardiovascular events.15
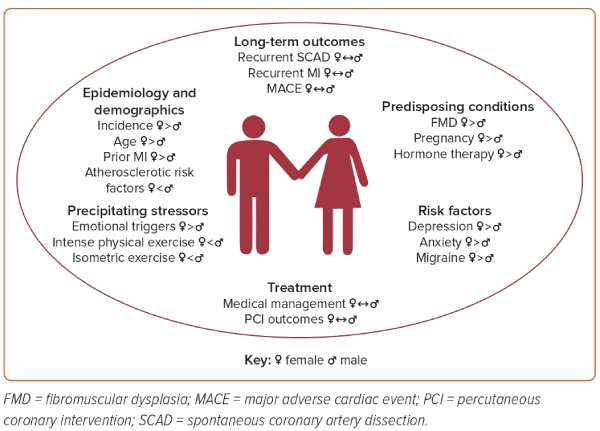
SCAD is the most common etiology of MI in pregnant patients, often presenting more severely and with a higher prevalence of ST-elevation MI (STEMI) than SCAD in patients who are not pregnant.17 Pregnancy-associated SCAD (p-SCAD) accounts for approximately 5–10% of all SCAD cases, with most events occurring in the third trimester and the early postpartum period.1,3,12 The prevalence of SCAD during pregnancy and the 6-week postpartum period is 1.81 events per 100,000 pregnancies in a US administrative database.18 Figure 2 further outlines the sex and gender differences and similarities in epidemiology, risk factors, and treatment for SCAD.
Clinical Presentation
Although there is a range of clinical presentations of SCAD, almost all patients have ACS symptomatology, with chest pain being the most common. A total of 2–5% of patients have cardiogenic shock.1 In a prospective cohort of 288 SCAD patients from Vancouver General Hospital, 72% of men and 70.4% of women reported precipitating factors prior to SCAD.19 In men, physical triggers occurred in 44.0% during isometric exertion involving >50 lb and emotional triggers occurred in 24% during emotionally stressful events, whereas women had emotional stressors prior to the event in 54.8% of cases and isometric exertion in 15.6% of cases.19 These findings have been replicated in a larger prospective analysis of the Canadian SCAD cohort involving 1,173 patients. Men were more likely to experience physical triggers including recent physical stress (men, 58.1% versus women, 45.5%), isometric physical stress (men, 40.2% versus women, 24%), intense isometric stress involving >50 lb (men, 25.6% versus women, 7.1%), and aerobic physical activity (men, 35% versus women, 30.1%).9,10 Women were more likely to experience emotional stress (women, 59.3% versus men, 35%) and chronic stress (women, 23.1% versus men, 14.5%).9,10 In a case series of 196 SCAD patients, 96% of patients reported chest discomfort upon presentation. Other common presenting symptoms included arm pain (49.5%), neck pain (22.1%), nausea and vomiting (23.4%), diaphoresis (20.9%), dyspnea (19.3%), and back pain (12.2%).20
Overall, the most common angiographic presentation is type 2 single-vessel SCAD, with multivessel disease occurring in approximately 10–40% of patients (Figure 1).16,21 There was no difference between the angiographic types of SCAD observed between men and women in larger cohort studies.10 This is different from previous findings from smaller studies and registries including the Massachusetts General Hospital SCAD registry, in which more cases of type 1 SCAD were seen in men.16,22 The most affected artery is the left anterior descending artery (57%), followed by the left circumflex artery (32%), right coronary artery (23%), and left main trunk (3%).21 Men had significantly more left circumflex artery dissections (44.4% versus 30.9%) and less frequent right coronary artery involvement (11.8% versus 21.7%) than women.9,10,21 In a review, all SCAD patients were diagnosed with troponin-positive ACS, with a median increase of 6 μg/l (measured with traditional troponin assays).1,23 ECG changes were also common in SCAD. A total of 26–87% of patients with SCAD had STEMI and 13–69% had non-ST-elevation MI (NSTEMI).1 Both men and women with SCAD presented with similar rates of STEMI. Overall, men were less likely than women to have ischemic ECG changes (49.6% versus 61.0%).10 The rate of in-hospital death from acute SCAD was 0.1%, that of severe ventricular arrhythmia was 3.9%, and hemodynamic instability, 2%.9 Only 1.4% of patients in this series required mechanical circulatory support due to cardiogenic shock.9 The rate of ventricular arrhythmia and that of reduced left ventricular ejection fraction (<50%) were similar between men and women.10
When comparing outcomes in SCAD patients by gender, McAlister et al. showed that recurrent chest pain was the most frequent presenting symptom after SCAD.10 Interestingly, men had significantly lower rates of chest pain than women at short-term follow-up (median, 49 days: men, 38.4% versus women, 50.2%).10 At long-term follow-up (median, 3 years), men also had lower rates of emergency department visits related to chest pain (men, 10.6% versus women, 24.8%) and hospitalization for chest pain (men, 0.8% versus women, 7.8%).10 There were no differences in the rates of recurrent MI, recurrent SCAD and major adverse cardiac events (MACE) between men and women.10
SCAD and Pregnancy
Pregnancy-associated SCAD is the most common cause of MI in pregnant persons and should always be considered as the etiology of chest pain or ACS in young peripartum or postpartum patients without risk factors for coronary artery disease.5 Efforts to understand the possible role of hormones in pregnancy and SCAD have led to extrapolations based on histological changes observed in the arterial composition of aortas from autopsy examination of pregnant patients.24,25 Relevant changes include fragmentation of reticulum and collagen fibers, loss of elastic fiber corrugations and lack of acid mucopolysaccharides typically beyond 32 weeks of pregnancy, even in the absence of comorbid arteriopathy.24,25 Additional histopathological changes shown in pregnant patients with SCAD include eosinophilic adventitial inflammatory infiltration and focal areas of mucinous microcystic changes in the vessel media, which may weaken arterial walls.26 These structural changes and hemodynamic fluctuations associated with pregnancy can result in intimal tears and increase the susceptibility for p-SCAD.27 The extent to which these histopathological changes exist beyond the duration of pregnancy is not known.
More than 70% of p-SCAD cases occurred in the postpartum period, typically in the first week.28 The prevalence of SCAD in pregnant patients presenting with anterior MI is between 15% and 43%.29,30 A retrospective study of 323 patients in the Mayo Clinic fibromuscular dysplasia (FMD) registry showed SCAD in 54 patients who were pregnant, miscarried or who were ≤12 weeks postpartum.28 The majority were multiparous with a history of infertility treatments, within 4 weeks postpartum and presented more acutely with high-risk features such as STEMI (p=0.009), left main SCAD (p<0.0001), multivessel SCAD (p=0.00027), and left ventricular function <35% (p=0.0071).9,28 The Canadian SCAD cohort identified peripartum SCAD as an independent risk factor for major in-hospital adverse events and 30-day MACE, which persisted during the 3-year follow up period (HR 2.3).9,31
Additionally, those with p-SCAD were less likely to have extra-coronary vascular abnormalities or arteriopathies such as FMD.28 The risk of recurrent p-SCAD with a prior history of dissections has not been well studied. A nested case–control study of 636 women of childbearing age found that most patients with a prior history of SCAD tolerated pregnancy without increased risk of SCAD recurrence compared with those with previous SCAD and no subsequent pregnancy.32 Given the unpredictable nature of SCAD and the minimal value of cardiovascular testing as surveillance to predict recurrence and increased severity of presentation associated with pregnancy, patients must undergo detailed preconception counseling to avoid unplanned pregnancies. There should be a detailed discussion of the natural history of SCAD, assessment of individual risk factors for recurrence, and plans for future pregnancy. Ultimately, these patients are advised to avoid further pregnancies after SCAD if possible.5
SCAD and Sex Hormones
Sex hormones have been implicated in the pathophysiology and development of SCAD given its predilection for women, including its strong association with pregnancy and peri-menopausal/menopausal period.5 However, the use of hormone replacement therapy (HRT) or contraceptives appeared to be similar in SCAD patients compared with the general population.5 The mechanism of estrogen in coronary arteries involves activation of nitric oxide (NO) synthetase in the vascular endothelium, leading to the production of NO and resulting vasodilation.12,25 This further decreases oxidative stress and promotes angiogenesis.12,25 It has been posited that progesterone counteracts the effects of estrogen (as described) in the coronary arteries.25
SCAD events have been shown to occur just before or during menstruation in premenopausal females, corresponding to the luteal phase of the menstrual cycle, and shortly after pregnancy in postpartum females.12,25 During these two time periods, the levels of circulating estrogen and progesterone typically decline, implicating ‘estrogen withdrawal’ or relative changes in the level of circulating estrogen and progesterone in females as a potential hormone hypothesis for SCAD.25 Interestingly, estrogen withdrawal has also been linked to coronary vasospasm and migraines; both associated with SCAD.25 In post-menopausal females and females who have undergone or are undergoing infertility treatment, the diagnosis of SCAD has also been temporally linked to initiation of HRT.5,25 Given the limited data on sex hormones in SCAD, patients are generally counseled against the use of exogenous hormones after a SCAD diagnosis.
SCAD and Genetics
Many extra-coronary arteriopathies with a genetic predisposition have characteristics that overlap with SCAD, often occurring in young patients without overt risk factors for cardiovascular disease. However, our understanding of SCAD genetics continues to evolve. The genetic traits for SCAD are now thought to be polygenic as opposed to monogenic or following a Mendelian pattern, as with other previously characterized arteriopathies.33 SCAD incidence has been described in association with genetically triggered and familial connective tissue diseases (CTDs), such as Marfan syndrome, vascular Ehlers–Danlos syndrome (vEDS), Loeys–Dietz syndrome (LDS), polycystic kidney disease, Alport’s syndrome, and aortic aneurysm.34–36 Overall, inherited arteriopathies and CTDs have been implicated in approximately 4–10% of SCAD cases.34
Several studies have investigated genetic patterns associated with SCAD and noted a heterogeneity in its genetic inheritance. In two small studies involving panel-based whole-genome sequencing of patients identified with SCAD, mutations associated with vascular disease, including COL3A1 seen in vEDS, PKD1 seen in adult polycystic kidney disease, and SMAD3 seen in LDS, were implicated.34,37,38
In a recent study involving genome sequencing in aortic aneurysms and dissection in SCAD patients with or without FMD, SMAD2 mutation was identified as a significant rare variant.32,38 Furthermore, studies using exome and whole-genome sequencing have identified additional pathologic variants. The largest sequenced cohort of SCAD survivors, with 384 patients compared with 13,722 controls from the UK biobank with data published in 2022, identified mutations linked with SCAD and CTDs, including FBN1, TSR1, TLN1, TGFB2, MYLK, and PHACTR1/EDN1.34,39,40
Although significant advances in genomics have enhanced our understanding of the genetics observed in SCAD, genetic testing of patients with SCAD is not routinely recommended in clinical practice due to unclear significance. In patients with physical exam findings, imaging, or family history suggestive of concurrent arteriopathy or CTD, genetic testing can play a larger role.
SCAD and Migraines
Migraine headaches have been linked to SCAD in addition to other vascular conditions, such as FMD, and are more prevalent in women affected by SCAD as compared with the general population. Several cohort studies involving patients with SCAD have reported a high prevalence of migraine headaches than in the general population. The Canadian SCAD registry reported a lifetime prevalence of 32.5% compared with approximately 24% of the general population.9 As mentioned above, a smaller percentage of men in the cohort reported a history of migraines compared with women.9,10
A separate cohort of 585 patients in the Mayo Clinic multicenter SCAD registry found that approximately 40% of patients with SCAD also had a history of migraines, and of these, 99% were women.41 Women with SCAD and a history of migraine headaches presented with SCAD at a younger age and also had a high prevalence of depression, recurrent chest pain and vascular abnormalities including aneurysms, pseudoaneurysms and dissection than patients with SCAD and no history of migraine headaches.41 Another retrospective study evaluating patients with SCAD found a 3.4-fold increase in the risk of recurrent SCAD in patients with a history of migraine headaches.42
The treatment of SCAD and comorbid migraine headaches has clinical implications. Triptans are the most common medications prescribed to treat migraines and are contraindicated in SCAD patients. These medications cause vasoconstriction and vasospasm, which have been negatively linked to cardiovascular events and dissections.43 In patients with SCAD who have a history of migraines, these medications should be discontinued. β-blockers typically used for post-SCAD patients with chest pain and newer agents such as calcitonin gene-related peptide (CGRP) antagonists are safe and effective in this population. If needed, consultation with neurology is appropriate. Patients with SCAD and migraine headaches should be screened for comorbid depression in addition to vascular diseases.
SCAD and Arteriopathies
SCAD is often the first symptom of an underlying vascular disease.5 FMD is a non-atherosclerotic, non-inflammatory vasculopathy most commonly associated with SCAD.1,3 It is caused by dysplasia in medium-sized arteries causing stenoses, tortuosity, aneurysms, and dissections.1,44 FMD is multifocal in >70% of cases, defined as alternating areas of stenosis and dilatation causing a ‘string of beads’ appearance, compared with unifocal FMD, which appears as an area of focal stenosis.3,5
The prevalence of FMD in patients with SCAD ranges from 45% to 72%, with recent literature suggesting a prevalence greater than 50%.5,9 More than 90% of FMD cases occur in women at a mean age of 50 years old, affect mostly renal, iliac, and cerebrovascular beds, and rarely affect coronary arteries.9 Few case reports have shown coronary FMD in SCAD patients after post-mortem histopathological analysis of coronary vessels.45
In a 3-year follow-up of a Canadian SCAD cohort, fewer men had FMD than women (27.8% versus 52.7%) overall.9,10 It is worth noting that the prevalence of FMD in men with SCAD in this cohort was higher than in the general population of men.9,10 SCAD can be associated with vascular tortuosity, aneurysms, or dissections affecting both coronary and extra-coronary arteries in the absence of FMD.33
Other systemic arteriopathies that may present with SCAD include Marfan syndrome, vEDS, and LDS. The co-prevalence of SCAD with vascular abnormalities can have specific implications in women, especially those of childbearing age who are pregnant or contemplating future pregnancies.5 Screening and surveillance of systemic arteriopathies, specifically FMD, should be performed in SCAD patients using non-invasive head-to-pelvis cross-sectional imaging with CT angiography or magnetic resonance angiography, with the goal of identifying clinically relevant vascular complications including but not limited to intracranial and extra-coronary aneurysms or dissections that warrant further surveillance or treatment.5 If FMD is diagnosed, there should be further discussion regarding prognosis, surveillance, and counseling for potential vascular complications.
SCAD and Acute Management
Management in the acute phase of SCAD depends on the clinical severity, and the goal is to preserve cardiac function and myocardial perfusion.5 Patients without high-risk features such as persistent chest pain, ongoing ischemia, hemodynamic instability, ventricular arrhythmias, left main dissection, or extensive proximal multivessel SCAD are considered low risk and should be managed conservatively.1,5,9,46 Patients who present with acute SCAD might receive initial treatment according to the ACS algorithm, which includes the use of dual antiplatelet therapy (DAPT), intravenous heparin, and β-blockers.1,5 In a recent prospective study, the majority of SCAD patients were treated conservatively with medical management consisting of antiplatelet therapy and β-blockers.9 There was ultimately no significant difference in the rates of conservative treatment between men and women.9,10
Although percutaneous coronary intervention (PCI) is recommended by guidelines for patients with atherosclerotic MI, it is generally avoided in acute SCAD and reserved for high-risk patients, given the risk of further iatrogenic dissection or extension of previous dissection, vessel occlusion and hematoma propagation.1,5,9,46 Indications for PCI in acute SCAD may include active or ongoing ischemia, ventricular arrhythmias, and hemodynamic instability.1,5 There are no standardized criteria for determining successful PCI in acute SCAD patients, but the factors that can be considered include the degree of stenosis and presence or absence of residual dissection.46 Men and women had similar rates of success and complications after PCI.10
Coronary artery bypass grafting (CABG) is often reserved for high-risk patients with left main or proximal multivessel dissections or instances of PCI failure.1,5,46 In the Canadian SCAD cohort, 86.4% of patients presenting with SCAD were initially managed conservatively and of those, 2.0% required PCI during the index admission and 0.4% received CABG.9 The rate of in-hospital adverse events in this population was rare and similar between men and women.10 Notably, the median hospital stay was 4 days, emphasizing the need for adequate monitoring of patients prior to discharge.46 Acute SCAD occurring during pregnancy should be managed similarly as above without compromising maternal and fetal outcomes.5
SCAD and Medical Therapy
Currently, no randomized controlled trials exist to guide medical management of SCAD, although some are currently ongoing. In stable SCAD patients, conservative medical treatment is preferred given that the affected vessels are expected to heal spontaneously.1,5 This involves the initiation of β-blockers and antiplatelet agents, which reduce shear stress and shear-mediated platelet aggregation on the coronary vasculature, respectively. The main goal is to relieve symptoms including chest pain, prevent recurrent SCAD, and manage hypertension. Data showing benefits of β-blockade in SCAD patients are based on a 2017 cohort study of patients in Vancouver.31 The study showed diminished rates of recurrent SCAD in patients using β-blockers.31 There are different practices with regards to the use of antiplatelet therapy after SCAD, some of which remain controversial. DAPT may be used in the short term (2 weeks–3 months) or long term (up to 1 year) prior to transition to low-dose aspirin monotherapy in patients managed conservatively, although there is no evidence to support this.1,5,47 Other options include the use of low-dose aspirin monotherapy alone.1,5,47
In SCAD patients in the European DISCO multicenter registry treated with low-dose aspirin versus aspirin and clopidogrel or ticagrelor, a higher MACE rate was observed in patients who received DAPT.48 The BA-SCAD randomized trial is an ongoing pragmatic trial looking into the benefits of initiating β-blockers and short-term versus long-term antiplatelet therapy with DAPT on a composite outcome including death, MI, stroke, coronary revascularization, recurrent SCAD, or hospital admissions.49 The results of this study will provide crucial insights into current medical management practices for SCAD.
Patients with diminished left ventricular function after SCAD should be placed on appropriate four-pillar guideline-directed medical therapy in addition to β-blockers, including sodium–glucose cotransporter 2 inhibitors, mineralocorticoid receptor antagonists, and either angiotensin receptor/neprilysin inhibitors, angiotensin receptor blockers (ARBs), or angiotensin-converting enzyme inhibitors (ACEIs).1,5
High-risk patients who have required PCI with stent placement should be treated according to current guidelines for coronary artery revascularization.50 There is no absolute indication for statin initiation in patients with SCAD. Use should be restricted to those who meet guideline criteria for primary prevention of cardiovascular disease.2 Interestingly, recent prospective data of SCAD patients found that the use of medications, including β-blockers, aspirin, and P2Y12 inhibitors, did not predict 3-year MACE outcomes.9 There was also no difference in the prescription of aspirin, β-blockers, clopidogrel or ticagrelor, ACEIs or ARBs, and calcium-channel blockers at discharge between men and women with SCAD.10 In our practice, patients who present with SCAD and do not require PCI are treated conservatively with aspirin monotherapy and β-blockers as tolerated.
SCAD and Counseling
Prior to discharge and upon outpatient follow-up, it is crucial to counsel patients on appropriate lifestyle changes, activity modifications, and medications to lower the risk of recurrence. Physical activities, including high-intensity exercise, Valsalva maneuvers, and heavy weightlifting, should be avoided.6 During cardio-aerobic exercise, a heart rate up to 75% of the patient’s calculated maximum heart rate has been previously shown to be safe.51
Additionally, all patients should be referred to cardiac rehabilitation, which may be SCAD-specific, on discharge.6 Cardiac rehabilitation along with other aerobic physical activity can improve exercise capacity and mental health, and reduce chest pain.6
Conclusion
With increased awareness of SCAD, we remain faced with the challenge of refining current practice to improve outcomes and reduce recurrence. Understanding sex and gender differences in SCAD provides further insights. Women with SCAD are typically older and likely to have emotional triggers compared with men. Comorbid conditions in women include a history of migraine, depression, and anxiety. Common predisposing risk factors specific to women include pregnancy and FMD. Clinical and angiographic presentation, treatment and rates of MACE are similar between men and women. Counseling is crucial for women with SCAD and different comorbidities as discussed above. Gaps in knowledge remain around genetics, sex hormones, reproductive and mental health implications, and management. 
Clinical Perspective
- Spontaneous coronary artery dissection (SCAD) is a common cause of acute coronary syndrome in young, middle-aged, and postpartum women.
- Morbidity and mortality rates following an acute SCAD event are low.
- Fibromuscular dysplasia and pregnancy are the most significant predisposing factors for SCAD in women.
- Post-SCAD counseling should include discussions regarding recurrence risk, reproductive implications, physical activity precautions, possible psychological effects, and referral to cardiac rehabilitation.
- There is ongoing research regarding optimal medical management for SCAD.










