Acute MI (AMI) remains the number one cause of inpatient mortality. With advancements in therapy, mortality remains <5% in the current day and age.1 Effective, complete, and timely reperfusion with percutaneous coronary intervention, when feasible, remains the cornerstone of therapy in AMI.2,3 Not uncommonly, AMI can be complicated by cardiogenic shock (CS), leading to hemodynamic instability, multiorgan failure, and escalated mortality. The presence of CS can make revascularization challenging during the AMI setting. The data from the CULPRIT-SHOCK trial support culprit-only revascularization in the AMI-CS setting with a primary reduction in 30-day mortality and renal replacement therapy.4
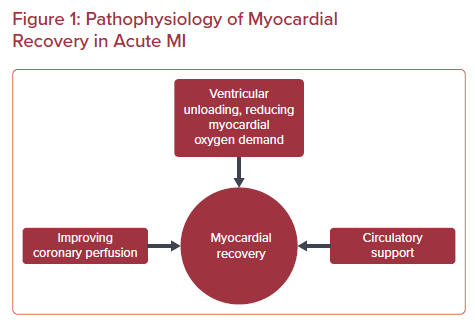
Despite advancements in therapeutics, mortality in AMI complicated by CS is exceptionally high.5 CS complicating AMI remains the leading cause of AMI mortality. Predominantly, in over two-thirds of cases, left ventricular (LV) failure is the leading etiology of CS.6 Other etiologies include mechanical complications, such as mitral regurgitation, free wall rupture, and acute right ventricular failure, among others. Mechanical circulatory support (MCS) devices are often used in CS complicating AMI, despite a lack of sufficient randomized data demonstrating mortality benefits.7 In theory, the ideal MCS device should intend to unload the myocardium, halt the spiral of ischemia, prevent hypotension, allow for adequate reperfusion, and aid myocardial recovery (Figure 1).
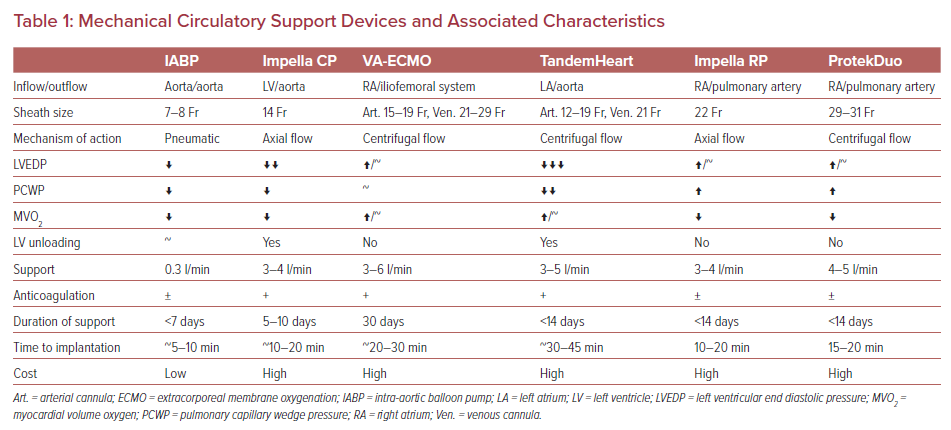
Various disparities exist in the usage of MCS devices and their effects on clinical outcomes.8 Several registry data have identified improved outcomes using early recognition of CS, timely escalation, invasive hemodynamic monitoring, and systematic/algorithmic approaches to device selection for patients with AMI-CS. Severe percutaneous MCS support devices are available for use in the AMI-CS setting. They are summarized in Table 1. In this review paper, we will discuss the commonly used MCS devices in the AMI setting.
Intra-aortic Balloon Pump
Since its inception in the 1960s, the intra-aortic balloon pump (IABP) has been the most widely used mechanical device in AMI.9–11 It is employed as an adjunctive measure for high-risk percutaneous coronary intervention (PCI)/coronary bypass artery grafting and/or to provide hemodynamic support for cardiogenic shock following AMI.12 IABP counterpulsation works by augmenting diastolic blood pressure to increase coronary blood flow. The balloon is deflated in pre-systole, which reduces afterload, hence reducing myocardial oxygen consumption and stroke work.13 IABP is typically placed in femoral arteries, but can be placed via axillary or brachial arteries, using an 8 Fr sheath. Inflation during diastole augments diastolic pressure with an expected increase in coronary blood flow and systemic perfusion. While multiple human and animal studies with IABP have demonstrated a decrease in afterload and systemic acidosis with an increase in cardiac index, myocardial blood flow, and systemic perfusion, findings are not consistent across patient groups, and studies evaluating patient outcomes report mixed results, calling the utility of IABP into question.14–22
Several studies have demonstrated no survival benefit from IABP in the AMI setting, regardless of the presence of CS.20,23 The 2008 European and 2009 US guidelines featured class I recommendations for the use of IABP in AMI-CS.24,25 These recommendations were largely based on the mortality benefits of IABP observed among AMI patients treated with thrombolysis before PCI became widely used.21,22,26–28 However, the SHOCK trial from 2012, which was the first prospective randomized trial evaluating the application of IABP among AMI-CS patients treated with PCI, failed to demonstrate improvement in hemodynamic indices (outside of pulmonary capillary wedge pressure) and severity of CS with the addition of IABP compared with vasopressors alone.19 This was followed by the larger SHOCK II multicenter trial, where IABP was not associated with differences in the degree of CS and mortality among AMI-CS patients treated with early PCI.20 These findings led to the 2013 US and 2014 European guidelines downgrading the recommendation for use of IABP in AMI-CS to class IIa and III, respectively.29,30 A meta-analysis evaluating the use of IABP in AMI-CS for reperfusion strategy revealed IABP to be associated with no difference of in-hospital mortality in the absence of reperfusion, a significant risk reduction of in-hospital mortality with thrombolysis, but a risk increase with concomitant PCI.10 Follow-up meta-analyses further revealed no difference in mortality, ischemic events, and bleeding rates associated with IABP among AMI-CS patients across randomized controlled trials (RCTs) and observational studies.23,31
While the routine use of IABP for AMI-CS is no longer recommended, contrasting reports have left room for debate regarding its use in AMI-non-CS (NCS). Given the widespread use of PCI for AMI, there are limited studies evaluating the use of IABP without PCI in AMI-NCS. Two small observational studies among AMI-NCS patients undergoing thrombolysis showed improvement in mortality in the IABP group.32,33 However, one RCT of AMI-NCS treated with thrombolysis found no difference in functional class and mortality associated with IABP.34 These studies are reflective of the pre-PCI era, and further studies evaluating the use of IABP with thrombolysis may not be needed, given the wide availability of PCI. Extrapolating from the modest benefit observed with the use of IABP in AMI-CS treated without reperfusion or thrombolysis alone, IABP may be considered if emergent percutaneous or surgical revascularization is not an option, or if clinical or logistics do not support the use of other mechanical support devices.10,21,22,26,28,35–37
Among patients with AMI-NCS with concomitant PCI, three RCTs have reported no improvement in mortality, re-infarction rate, coronary blood flow, and ventricular function with IABP placement following PCI, along with one RCT with prophylactic IABP in ST-elevation MI patients demonstrating no decrease in infarct size.18,38–40 In contrast, Ohman et al. demonstrated prophylactic IABP to be associated with reduced ischemic events and re-occlusion of the infarct-related artery, with Gu et al. reporting a decrease in troponin I, C-reactive protein, and 30-day mortality.41,42 These discrepant results raise the potential concern of the timing of IABP placement as being an important factor. However, the potential benefits of IABP must be balanced with a higher risk of bleeding.31
Due to ease of placement and widespread availability, IABP remains the most commonly used MCS device in the AMI setting. Overall, there is a considerable discrepancy regarding the benefit of IABP in AMI. Meta-analyses have not revealed a mortality benefit across the entire aggregate of IABP-treated patients or among subgroups of AMI-CS and AMI-NCS.10,23,31 Across studies of AMI-CS patients, IABP appears to confer a mortality benefit when reperfusion with thrombolysis is pursued, whereas an increase in mortality is apparent with concomitant PCI.10 The same trend was again observed in a meta-analysis of observational studies evaluating the use of IABP in AMI with respect to reperfusion strategy regardless of shock status.23 For application in current clinical practice, while the lack of a mortality benefit of routine IABP is clear for AMI-CS treated with PCI, patient-specific use of IABP may still serve as a bridge to complex revascularization or more advanced mechanical support devices. For AMI-NCS undergoing PCI, evidence regarding IABP is less clear, and further randomized studies with prophylactic IABP are needed to clarify the benefit for this subgroup.
Impella Support Pump
Impella (Abiomed) is a mechanical circulatory support device that can be implanted percutaneously or surgically via the femoral and axillary artery or surgically via an aortic cutdown. The Impella support pump is advanced via a transaortic approach into the LV, and helps support LV function and ventricular unloading.43 Impella support devices are continuous, non-pulsatile, axial pumps that are available in various configurations, that incrementally provide hemodynamic support, and are used on the basis of the support required and ease of insertion. These include Impella 2.5, Impella CP, Impella 5.0, and Impella 5.5 devices, the latter two requiring surgical cutdown for insertion. Data suggest that Impella use is associated with reduced infarct size in the AMI setting.44 In addition to augmenting cardiac output, the device helps in LV unloading, reducing myocardial oxygen consumption, and improving coronary and systemic perfusion by increasing forward flow.45 No randomized data have shown the mortality benefit of Impella over other support devices in the AMI setting.
The IMPRESS in Severe Shock trial was a randomized, open-label study that failed to show mortality benefit in AMI-CS patients receiving Impella support compared with IABP support.46 Benedikt et al. retrospectively evaluated the AMI-CS patients with the IABP-SHOCK II matched cohort and found no difference in 30-day mortality in the Impella arm (48.5% versus 46.4%; p=0.64). Severe, life-threatening bleeding and vascular complications were higher in the Impella arm.47 The data from PROTECT II trial suggest that patients with complex multivessel disease and reduced LV ejection fraction, or unprotected left main coronary artery, had a similar composite primary endpoint of 30-day incidence of 11 major adverse events, which was similar between the Impella and IABP groups (35.1% for Impella 2.5 versus 40.1% for IABP; p=0.227). There was a trend toward reduced incidence of adverse outcomes at 90-day follow-up in the Impella arm.48 This study, however, included patients undergoing PCI in non-emergent settings, questioning its validity in the AMI setting.
Traditionally, delayed revascularization has been proposed as a predictor for adverse outcomes in AMI patients. However, experimental data suggested that ventricular unloading using an Impella device before revascularization reduced infarct size at 28 days after AMI.49 Registry data suggested survival benefits in pre-procedure Impella placement in AMI-CS patients and high-risk PCI without CS.50 Kapur et al. demonstrated the feasibility of Impella placement before attempting revascularization in an anterior ST-elevation MI setting.51 In a small study of 88 patients in an AMI-CS setting, the placement of pre-PCI Impella versus post-PCI Impella did not show any significant mortality differences among the groups.52 The ongoing Door to Unload trial (NCT03947619) should provide more insights into the widespread clinical application of Impella in this setting.
The National Cardiogenic Shock Initiative advocates a protocolized approach in treating AMI-CS patients, with excellent reported survival rates of 72% at 30 days.53 Analysis from real-world data using the Premier Healthcare Database showed a higher degree of adverse outcomes and costs in patients undergoing PCI with MCS.54 Compared with IABP, Impella use was associated with a higher risk of death (OR 1.24; 95% CI [1.13–1.36]), bleeding (OR 1.10; 95% CI [1.00–1.21]), and stroke (OR 1.34; 95% CI [1.18–1.53]). The heterogeneity of the population, selection bias, and usage of administrative databases limit the generalizability of the study.
Nevertheless, controversy exists regarding the appropriate use, timing, and mortality benefit of Impella in AMI patients. In patients with AMI and associated mechanical complications, the Impella can provide necessary support until definite recovery or destination therapy. Ventricular septal rupture post-MI is associated with high mortality, especially if there is an attempt at early repair, compared with a late repair after 7 days.55 Impella devices are useful to provide mechanical support to these patients until the transition to definite surgical repair.56,57
Impella device complications are not rare, and that can halt the overall hemodynamic benefit. The Impella support pumps require meticulous care during insertion and management afterward. Careful access selection and vessel sizing are critical to prevent vessel injury and any distal extremity vascular compromise. Bleeding, hemolysis, and thrombocytopenia can be drastic device-related complications, which can affect the safe administration of anticoagulation and antiplatelets. That device support must be de-escalated and discontinued promptly when clinically feasible to avoid device-related complications.
Extracorporeal Membrane Oxygenation
Extracorporeal membrane oxygenation (ECMO) devices, originally designed to function as cardiopulmonary bypass circuits, provide robust hemodynamic and respiratory support by giving cardiac output over 3–6 l/min, depending on cannula size, and assisting in gas exchange. Blood is taken from the venous system and returned to the arterial system via the ECMO cannula system. Veno-venous, veno-arterial, and various other configurations can be used to support the patient based on the requirement for a particular case. ECMO requires extensive care, including perfusionists and dedicated nurses, halting widespread availability except in tertiary care centers.
Unlike some other MCS devices, one potential effect of VA-ECMO support devices is an increase in afterload that may halt myocardial recovery in the AMI setting.58 Some advocate the use of unloading devices in these settings, such as ECMO and Impella; that is, ‘ECAPELLA’ support. Some authors have suggested using ECMO-IABP for unloading; however, comparative studies show higher survival in the ECAPELLA group compared with the ECMO-IABP group (365-day mortality 43.5% versus 75.6%, respectively; p=0.010).59
Poor neurological outcomes and high mortality rates are associated with patients who present with cardiac arrest in the setting of AMI. ECMO support has been used for ECMO-assisted cardiopulmonary resuscitation for a patient who presents with cardiac arrest in the setting of AMI. Timely usage of ECMO-assisted cardiopulmonary resuscitation followed by early reperfusion in these patients has been shown to have good clinical neurological outcomes.60
ECMO support in AMI is generally used for a patient in advanced shock, biventricular failure, AMI with mechanical complications, and in patients who require ECMO support for concomitant respiratory failure. A large meta-analysis of ECMO use in AMI showed high short-term mortality of 58%, and a high rate of bleeding, renal failure, and neurological damage.61 ECMO requires a large-size cannula to provide adequate support, hence increasing the risk of vascular injury and bleeding. ECMO carries a higher risk of coagulopathy, circuit thrombosis, vascular injury, limb ischemia, air embolism, and pump failure, as compared with other MCS devices. Routine use of ECMO in the AMI setting is limited due to the risk of increased myocardial work and higher device-related complications.
TandemHeart
TandemHeart (CardiacAssist) uses a centrifugal pump with a fluid dynamic hydraulic bearing to divert the blood flow from the left atrium to the iliofemoral arterial system.62 Unlike ECMO pumps, the TandemHeart unloads the LV by decreasing LV end diastolic pressure (LVEDP) and, in turn, reducing myocardial oxygen consumption, accelerating recovery. At higher flow rates, the transaortic blood flow will compete with the flow in the output arterial cannula, and the LV unloading effect may be reduced.
The safety and efficacy of TandemHeart were compared with IABP for CS patients, 70% of which was related to AMI. The study found no difference in mortality at 30 days despite improvements in pulmonary capillary wedge pressure, mean arterial pressure, and cardiac index.63 The TRIS trial (NCT021464058) was proposed to assess the impact of LV unloading, but it was terminated in 2015 due to a lack of enrollment. Routine use of TandemHeart in AMI-CS is limited due to the availability of other easily used devices, challenges involving insertion using transseptal puncture, complex management post-implantation, and risk of complications.
Right Ventricular Support
Right ventricular (RV) failure is a disastrous complication of AMI. Medical management involves volume expansion, ionotropic support, maintenance of atrioventricular synchrony, and RV mechanical support. The RV support devices can be isolated percutaneous RV support devices, such as micro-axial flow pumps and extracorporeal centrifugal flow RV assist devices, surgically implanted RV assist devices, and VA-ECMO.
Impella RP is 22 Fr and is mounted on an 11 Fr catheter, inserted via femoral approach into a pulmonary artery. The physiological concept is similar to the LV Impella pump, involving unloading the ventricle and, hence, reducing the oxygen consumption, allowing time for myocardial recovery. It directly bypasses the RV and directs blood from the right atrium into the pulmonary artery. It can increase LV preload and cardiac output. The RECOVER RIGHT trial studied Impella RP use in RV failure, including over one-third of patients with AMI.64 The study showed 30-day survival of 73%.
ProtekDuo is inserted using single access from the internal jugular vein, allowing for mobilization of the patient. The distal outflow port of the device enters the pulmonary artery and drives blood from inflow from the right atrium. It can provide up to 4–5 l/min of blood flow. VA-ECMO is a unique RV support device, as it provides both RV and LV support, and is useful for patients with AMI complicated by biventricular failure and CS. Evidence to support the use of VA-ECMO for mortality benefit in patients with RV failure in the setting of AMI is lacking. The critical lifesaving step in the AMI patient with RV failure involves early recognition of RV failure, hemodynamic monitoring, and timely escalation for patients who are candidates for RV support.
Conclusion
Appropriate device selection is key to the successful management of the patient in AMI that requires MCS. It is important to recognize any potential condition that may favor the use of one device over another. RV failure can complicate AMI and may compel the use of RV support devices. Early recognition of CS, and timely insertion and escalation of MCS are critical for good patient outcomes.