Significant mitral regurgitation (MR), frequently seen in the presence of severe aortic stenosis (AS), results in an association that negatively affects prognosis and imposes particular challenges for both the assessment of the severity of valvular lesions and decisions regarding treatment allocation. Significant MR (Grade ≥2) is present in 25–30% of patients treated with transcatheter aortic valve replacement (TAVR), whereas severe MR (Grade 4) is seen in 2–5%.1
Here, we review the available literature with regard to assessing MR and AS when both are present, surgical results in patients with concomitant AS and MR, the effects of MR on TAVR patient outcomes, and the effects of TAVR on MR severity. Our aim is to provide assistance with decisions to treat patients with either a higher-risk double-valve procedure or a simpler, but perhaps incomplete, single-valve option.
Patients presenting with multivalvular disease (MVD) are common and often present with heterogeneous valve defects. The Euro Heart Survey and the EURObservational Research Program Valvular Heart Disease Registry demonstrated that one-fifth of patients with native valve disease have MVD, with AS and MR being the most common association.2 The 2021 European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines recommend that patients presenting with severe primary MR, undergo mitral valve surgery at the time of surgical aortic valve replacement (SAVR).3 In patients with severe secondary MR, surgery may also be considered in the presence of significant annular dilatation and marked left ventricle (LV) enlargement. In high-risk or inoperable patients with severe AS and severe MR, combined (or more often sequential) transcatheter aortic valve implantation (TAVI) and transcatheter edge-to-edge repair (TEER) may be feasible, but there is insufficient experience to allow robust recommendations.3 The 2020 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines on MVD stated that, overall, patients with severe AS and severe primary MR are best treated with SAVR and mitral valve surgery unless the surgical risk is high or prohibitive.4 If there is a high or prohibitive surgical risk, a staged procedure with TAVI followed by mitral TEER can be effective.4 If there is severe AS and severe secondary MR, either SAVR and mitral valve surgery or a staged approach with TAVI followed by mitral TEER are options.4
Assessment of Valvular Lesions in the Setting of Multiple Valve Disease
Evaluation of the severity of valvular lesions is an obvious critical step in patient management. Only with precise diagnosis can clinicians provide accurate guidance and treatment options. In the setting of MVD, identification of the most significant or clinically relevant valvular lesions is paramount in the search for the most appropriate treatment. Although there are clear recommendations from international societies for the diagnosis of severe AS and MR, the hemodynamic and structural consequences of MVD often blur the evaluation of a single valvular lesion.5
Although rheumatic disease has historically been the most frequent cause of MVD, a shift towards degenerative disease is seen in developed countries.6
Assessment of Mitral Regurgitation in the Presence of AS
A transthoracic echocardiogram (TTE) remains the cornerstone of valvular disease diagnosis. A TTE provides insights into the cause of MR, as well as quantitative and semiquantitative assessment of MR severity.
MR etiology, reliably identified with TTE and/or transesophageal echocardiography, can be divided in primary (or degenerative/organic), secondary (or functional), or mixed.7 Degenerative MR is associated with calcification of the mitral apparatus, ruptured chordae, flail leaflets, myxomatous degeneration, or a combination of these findings. However, secondary MR is common in AS patients in whom a high prevalence of coronary artery disease, LV remodeling, and/or AF result in mitral leaflet tethering, annular dilation, or both. The potential of MR regression after relief of AS seems greater when MR is functional.7–9
The severity of MR is typically assessed with TTE using semiquantitative Doppler color flow jet area and quantitative measures such as vena contracta, proximal isovelocity surface area, and regurgitant volume or fraction. The increased systolic LV pressure inherent to severe AS results in a higher systolic transmitral gradient, which consistently increases the color flow jet area, resulting in an overestimation of MR.10 In addition, left atrial enlargement cannot be used as a surrogate to diagnose severe MR when AS-induced LV hypertrophy is present. Although AS added to MR does increase regurgitant flow at any orifice area, estimated regurgitant orifice area and vena contracta are less affected by higher velocities than color flow jet area and should be relied upon in the presence of AS.11 A high color flow jet area but small vena contracta and/or estimated regurgitant orifice area in the setting of AS probably reflects non-significant MR. Regurgitant volume and fraction can reliably assess MR severity in the presence of AS, but degenerative changes in the mitral annulus can lead to imprecise mitral inflow orifice estimation.12
Cardiac magnetic resonance is emerging as a reliable tool to assess MR, providing quantification of regurgitation volume based on LV volumes and aortic flow quantification.13 However, this volumetric method is of limited value when both the aortic and mitral valves are regurgitant.13
Assessment of Aortic Stenosis in the Presence of MR
MR reduces forward flow, resulting in lower transvalvular aortic velocity and gradient, even more so when AF occurs. Not only does paradoxical low-flow severe AS present commonly with severe MR, but also the afterload reduction provided by MR can shadow early detection of LV systolic dysfunction. In the absence of high velocity, TEE diagnosis of severe AS relies on calculation of the aortic valve area (AVA), which may be imprecise due to operator-dependent, technically limited estimation of the outflow tract area. TTE or transesophageal echocardiography planimetry of the aortic valve is of limited value if MR is severe and forward driving pressure is reduced.3
Aortic valve calcium scores from multidetector CT (MDCT) are strong predictors of severe AS with most discriminative cut-off scores for severe AS of >2,000 arbitrary units (AU) in men and >1,200 AU in women.3,14 Aortic valve calcium scores are highly reproducible and should be used when uncertainty remains after echocardiographic assessment. Alternatively, 3D measurements of the non-circular outflow tract using 3D echocardiography or MDCT associated with conventional Doppler flow quantification represents an intriguing method that could provide an accurate calculation of the AVA.15 However, current TEE-based threshold for severity (AVA <1 cm2 or indexed AVA <0.6 cm2/m2) may not apply to this method when most 3D-derived measurements result in a larger outflow tract area than that provide by 2D TEE.15
Right and left heart catheterization is recommended when TEE is non-conclusive in the setting of MVD.15 However, the intricacies of MVD also apply to invasive evaluation. Measurement of cardiac output using thermodilution is unreliable in the presence of severe tricuspid regurgitation or very low output, and an estimate of oxygen consumption is used in the Fick method. In addition, the Gorlin formula for calculation of valve areas cannot be used for valves that are both stenotic and regurgitant. Therefore, heart catheterization should be performed meticulously and the results interpreted with caution in patients with MVD.
For patients who can safely exercise, hemodynamic evaluation at a higher-flow state can provide discriminatory, yet poorly proven information.5 When stress induces changes that are a hallmark for a specific lesion, such as a high gradient for AS or markedly increased pulmonary arterial pressure for MR, clinicians may be tipped toward the culprit lesion.5
With all the limitations surrounding typical TEE evaluation of AS and MR in the presence of each other, it is necessary to integrate all information. Using unconventional assessment tools may also provide useful additional information.
Surgical Management and Outcomes of Combined Aortic and Mitral Valve Disease
Combined surgical procedures on multiple valves are associated with increased operative risk. Risk stratification models provide mortality estimates only for specified procedures and, for example, the Society of Thoracic Surgeons (STS) risk assessment tool, namely the Predicted Risk of Mortality (PROM), does not include estimates for concomitant aortic and mitral valve surgery.16 The 2008 STS registry unadjusted short-term mortality for all isolated valve procedures was 3.4%, whereas in-hospital morbidity rates ranged from 0.3% for deep sternal wound infection to 11.8% for prolonged ventilation.16 However, 2013 data from the STS show perioperative mortality after aortic and mitral valve operation almost threefold higher (9.4%) than that after isolated aortic valve replacement (AVR; 3.2%).17 In 2016, outcomes of left-sided valve replacement with modern prostheses in patients from a large single center study were published.18 In that study, in which the mean (±SD) age of patients was 74 ± 7 years, the mean (±SD) 30-day survival rate for patients with combined AVR and mitral valve replacement (MVR) was 92.8 ± 1.6%, compared with rates of 97.3 ± 0.4% and 95.1 ± 1.2% for those with isolated AVR and isolated MVR, respectively.18 The mean (±SD) 10-year survival rates for patients with AVR+MVR, AVR, and MVR were 22.1 ± 7.1%, 42.1 ± 1.5%, and 33.9 ± 4.7%, respectively.18
The decision to operate on the mitral valve in the setting of severe AS depends on the severity and etiology of MR. In symptomatic patients with severe AS, valve replacement (conventional or percutaneous) is the treatment of choice, whereas in patients with severe MR, valve repair is generally favored over replacement if feasible, especially among patients with organic disease.19,20 In a large multicenter retrospective study, mitral valve repair (compared to MVR) was associated with lower perioperative mortality, improved survival, and better preservation of postoperative LV function.21 Although patients who underwent MVR were older and at higher risk, the performance of mitral valve repair was independently associated with lower mortality after adjustment for baseline characteristics. In addition, in the 2013 STS registry publication, perioperative mortality was 5.7% for MVR versus 1.6% for mitral valve repair.17 Among patients with severe functional MR of ischemic etiology, a multicenter randomized clinical trial revealed no significant differences in LV reverse remodeling or survival at 2 years among patients randomized to repair versus replacement.22 However, MR recurred more frequently in the repair group, leading to more heart failure-related adverse events and cardiovascular admissions.22 There is a general consensus that, for patients undergoing SAVR, a double-valve operation is indicated in the presence of severe MR.
However, the management of moderate MR at the time of SAVR is controversial.23 Studies focusing on MR evolution following surgical AVR are rare. In one retrospective study, moderate functional MR improved in 66% of patients after isolated AVR.24 The presence of congestive heart failure, an enlarged left atrium, and the degree of postoperative aortic insufficiency were independent predictors of lack of improvement. In the PARTNER trial, 59 of the 299 high-risk patients who underwent isolated SAVR had moderate (90.5%) or severe (9.5%) MR.25 The overall mortality rate at 2 years was significantly higher among patients with moderate or severe MR (49.1% versus 27.9%; p<0.01), an association that remained significant after multivariate analysis (HR 1.77; 95% CI [1.17–2.68]).25
A meta-analysis of 17 studies and more than 3,000 patients published in 2011 confirmed that moderate–severe MR regurgitation adversely affects both early and late mortality following isolated AVR for severe AS despite MR regression in 60% of patients, reverse remodeling, and a reduction in LV end-diastolic diameter.26 In that meta-analysis, the overall unadjusted 30-day mortality was 5%, significantly lower (OR 0.41; 95% CI [0.24–0.72]) in patients without significant MR and remained significant when only patients with functional MR were analyzed.26 Although the authors suggested that concomitant mitral intervention should be considered in the presence of moderate MR, independent of etiology, the added risk of double-valve surgery was not taken into account in that review and the absence of adjustment for baseline characteristics limits the value of such a statement.
In contrast, a small, single-center, high-risk patient cohort of 43 patients with severe AS and significant MR undergoing combined AVR and mitral valve surgery suggests there may be no added benefit to mitral surgery.27 In that study, the mean (±SD) age was 80 ± 6 years, and the mean STS PROM was 10.1 ± 6.4%. Perioperative morbidity was 30% and the mortality rate at 6 months and 1 and 2 years was 25%, 35%, and 45%, respectively.27 Because the prognosis of the 43 patients in that study was similar to that of high-risk patients undergoing isolated TAVR, the authors concluded that, in such high-risk patients, there is no added benefit to a double-valve surgery.27
Impact and Evolution of Significant MR After TAVR
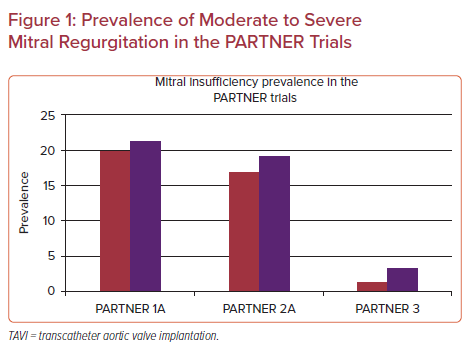
Although surgical data are scarce on the prognostic role of MR and the fate of untreated MR after relief of AS, the TAVR literature offers significant insights, because concomitant moderate or severe MR in patients undergoing TAVR has been reported to occur in approximately 20–30% of patients.25,28–31 The prevalence of significant MR decreases as patient risk profile improves, as evidenced by the proportion of patients with at least moderate MR in the different PARTNER trials (Figure 1). Correlations between functional MR and comorbidities and referral bias probably combine to explain this finding.
However, there are significant limitations that limit the strength of the available data:
- Severe MR is an exclusion criterion from TAVR randomized clinical trials.
- Quantitative MR evaluation is not reported in the vast majority of publications.
- Studies comparing outcomes of patients with and without MR have dichotomized patients based on different cut-off values for significant MR and include a small proportion of patients with truly severe MR, ranging from 2% to 9%, resulting in heterogeneity between studies and conflicting results.
- Modern TAVR devices and techniques may limit the generalization of older studies.
Baseline MR as a Predictor of Adverse Outcomes After TAVR
Multiple retrospective analyses and four meta-analyses have focused on the prognostic impact of significant MR after TAVI. The larger and most recent meta-analysis reported on 21 studies and more than 30,000 patients.31 Unadjusted short-term (RR 1.46; 95% CI [1.30–1.65]) and long-term (1–4 years in most studies included in the meta-analysis; RR 1.40; 95% CI [1.18–1.65]) mortality was higher for patients with MR.31 However, patients with significant MR presented with more comorbidities at baseline, including well-known adverse event predictors such as a higher STS score and lower ejection fraction (EF). Hence, 16 of 21 studies with 27,777 patients found no association between MR and mortality after adjusting for baseline variables. Interestingly, no interaction was found between studies that used MR Grade ≥2 or ≥3 as the dichotomization cut-off.31
However, a publication from the STS/ACC Transcatheter Valve Therapy Registry (TVT Registry) on TAVR procedures performed in 2012–13 suggests that the effect of MR on prognosis increases with MR severity (30-day unadjusted HR for mortality 1.27 and 1.84 for moderate and severe MR, respectively; 1-year unadjusted HR for mortality 1.30 and 1.46, respectively).32 After adjustment for baseline variables, HRs were no longer significant for mortality, but remained significant for the combined endpoint of mortality and heart failure hospital admissions.32 This gradual correlation between MR severity and 1- and 2-year mortality was corroborated by a recent single-center study.1
Earlier and smaller meta-analyses found similar effects of MR on 30-day and 1-year mortality.29–31 Baseline MR etiology did not have an effect on post-TAVR outcomes. In one of these publications that included 8,015 patients, significant MR at baseline persisted as a 1-year predictor of mortality after adjustment for baseline characteristics.30 Moreover, one retrospective multicenter study reported a threefold (35% versus 10.2%) increase in 6-month mortality in high-risk patients with significant MR despite no difference in baseline STS score or LVEF.8 That study is an outlier with regard to the level of effect of MR on prognosis, which could be related to methodological factors, including a more stringent (MR ≥3) definition of significant MR and the use of an echocardiographic core laboratory.
The association between significant preprocedural MR and mortality seems ever greater in the low-EF, low-gradient, severe AS. One study found that 1-year mortality was 3.5-fold higher in low-EF, low-gradient AS patients with moderate or severe MR than in low-EF, low-gradient AS patients who did not have significant MR (11.5% versus 38.1%, respectively; adjusted HR 3.27; 95% CI [1.31–8.15]; p=0.011).33
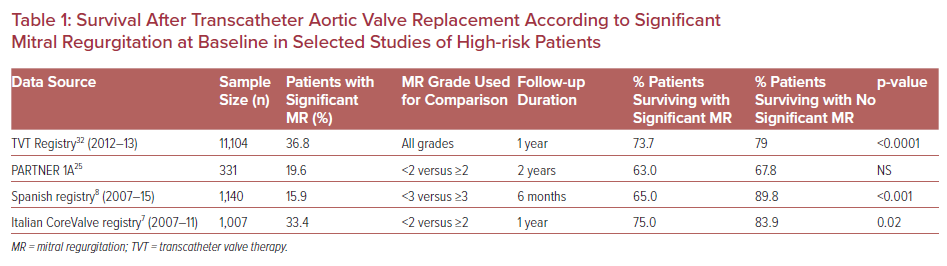
Despite this prognostic impact of significant baseline MR, high-risk patients with significant MR who undergo TAVR still derive benefit from the procedure, with acceptable mid-term survival (Table 1), improved quality of life, and New York Heart Association class in most patients.34,35
Changes in MR After TAVR
The etiology of MR in TAVR patients is more frequently reported as degenerative (approximately 50%) than functional (±30%), or mixed (±20%) in most publications. Improvement in MR of at least one grade is steadily reported in 50–60% of patients after TAVR.28–31 Such a low figure may be surprising given that AS relief uniformly leads to lower systolic LV pressure and transmitral gradient and probably underlines limitations associated with MR grading and/or lack of quantitative measurements. MR worsens after TAVR in approximately 2–7% of patients.36
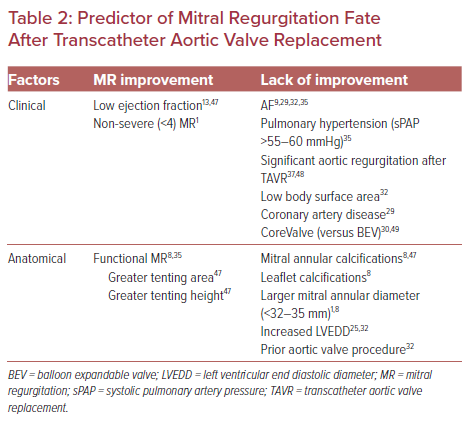
Many studies have aimed to identify clinical and anatomical factors that could predict MR improvement after TAVR. Table 2 presents the most frequently reported factors associated with MR improvement or lack of thereof. Degenerative MR, severity of mitral annular, and/or leaflet calcifications, as well as the presence of AF, were most often linked to persistent MR after TAVR.1,8,9,13,25,29,30,32,35,37,47–49
Some studies associated the use of a self-expandable transcatheter heart valve with more persistent MR after TAVR.29,30,37 Possible explanations for this include more residual aortic regurgitation contributing to LV volume overload, low implantation interacting with the mitral valve apparatus, and a higher likelihood of conduction defects. Transcatheter heart valve iterations limiting paravalvular regurgitation and reliance on higher implantation probably mitigate these findings in the current era.
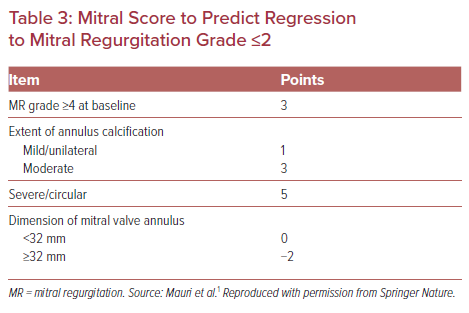
In a recent single-center study, Mauri et al. identified several anatomical factors associated with MR persistence after TAVR and created a “mitral score” (Table 3) to predict MR improvement.1 In their cohort, this score was highly discriminant for both MR persistence (ranging from 10.5% to 94.4%; AUC 0.816; p<0.001) after TAVR and overall prognosis (2-year mortality for score ≤4 versus >4, 47.8% versus 31.9%, respectively; HR 2.12; 95% CI [1.06–4.26]; log-rank p=0.030) following TAVR. Interestingly, baseline Grade 4 MR was the single most important predictor of persistent MR after TAVR.1 Although such a tool could be useful for patient assessment, to our knowledge external validation has yet to be published.
Impact of Residual MR on Prognosis After TAVR
MR regression is frequent after successful TAVR and may be attributed to several physiological changes, including lower transmitral systolic gradient, favorable LV remodeling, and regression of LV hypertrophy and end-diastolic volume leading to reduced tethering forces on the mitral leaflets. Many studies have assessed the impact of decreasing MR on prognosis, with the overwhelming majority finding that MR regression is associated with better prognosis.1,28,32,37,38 MR improvement to ≤2 often results in survival rates similar to the overall population. For example, in a recent single-center study of 677 consecutive TAVR patients, significant (≥3) MR at baseline was associated with lower 2-year survival (57.7 versus 74.4%; p<0.001), with those who experienced MR regression more likely to survive than those who did not (74.0% versus 54.1%; HR 2.02; 95% CI [1.43–2.86]).39 Patients with MR regression had an overall prognosis similar to the overall cohort (2-year survival 74.0% versus 77.8%, respectively).39 Data from the TVT Registry, SWEDEHEART, and other studies also suggest residual significant MR after TAVR is associated with worse outcomes, including increased deaths and heart failure admissions after adjustment for baseline variables.32,37 The adverse effect of MR seems gradual with increasing MR grade.38 One meta-analysis evaluated the impact of residual MR on survival and found higher mortality when MR persisted (RR 1.48; 95% CI [1.31–1.68]; p<0.00001).28
To our knowledge, only one study of 1,110 patients, including 157 with significant (MR ≥3), concluded that regression of MR, seen in approximately 60% of patients, was not associated with survival benefit.8
In summary, there is clear evidence that significant MR is associated with increased risk of acute and longer-term adverse events. However, it remains uncertain how much of this difference is attributable to MR or when MR is only a marker of worse prognosis. Significant MR improves at least one grade in approximately half the patients after TAVR, which can be predicted from clinical and anatomical variables. Lack of MR regression is linked to poorer prognosis.
Percutaneous Treatment of MR After TAVI
Percutaneous mitral valve repair using the MitraClip (Abbott Vascular) is associated with improved outcomes compared with conservative therapy in patients with symptomatic severe MR who are deemed high risk or inoperable.40 TAVI patients who remain symptomatic due to significant MR could potentially profit from a staged percutaneous procedure to treat MR. This may be a particularly attractive option for the subset of patients with low-gradient severe AS and moderate to severe primary MR, who tend to have a particularly high mortality after TAVR.33,36
When percutaneous treatment of AS and MR is contemplated, it is generally accepted that staging procedures, with TAVR first, is the most appropriate strategy. First, MR reduction in the setting of untreated severe AS is associated with a marked increase in afterload, which is likely to be poorly tolerated in patients who often have failing LV function, discouraging a strategy where the mitral valve would be addressed first. In addition, although single-stage TAVR with the MitraClip is possible, symptomatic improvement, MR reduction, and reverse remodeling of the LV after AS is relieved may mitigate the indication for a mitral intervention.
The first description of the MitraClip device being inserted as a staged procedure after TAVR with the Edwards and Medtronic devices was in 2011.41 Since then, three single-center series of patients undergoing staged TAVR and percutaneous edge-to-edge repair have been published, for a total of 37 cases with functional or degenerative MR.42–44 Procedural success was uniformly high, ranging from 91% to 100%. However, only two-thirds of patients survived to 1 year in two of those publications, with modest or no symptomatic improvement in most patients.42,43 The third publication only reported symptoms improvement at baseline and after both procedures, but a 100% survival at 6 months.44
Transcatheter mitral valve replacement (TMVR) seems closer than ever to enter clinical practice. However, as of now, there is little evidence for the use of mitral transcatheter valves in the mixed valvular disease population other than anecdotal, yet successful, cases of TAVR combined with mitral valve-in-valve implantation. A US National Registry publication (2014–18) identified that 0.1% of all TAVR patients underwent either mitral edge-to-edge repair (n=110) or TMVR (n=98) either as a combined or staged procedure.45 Compared with isolated TAVR, there was a fivefold increase in in-hospital death among patients undergoing TAVR and a mitral valve procedure (mortality 10.8% and 13.3%, with adjusted ORs of 3.87 and 4.34 for edge-to-edge repair and TMVR, respectively).45 Morbidity was also significantly more prevalent in patients undergoing aortic and mitral valve procedures in that publication.45 However, in another publication, a series of 12 high-risk patients with previous surgical AVR were treated with the Tiara (Neovasc) transcatheter valve via a transapical approach.46 Procedural success was 100%, with no death, MI, stroke, or major bleeding at 30 days. MR was eliminated in all 12 patients immediately after implantation. The authors of that study concluded that TMVR with the Tiara valve in high-risk patients with severe MR and previous AVR was technically feasible and safe.46
Based on these data in a small number of patients, transcatheter double-valve implantation seems currently best limited to highly selected patients and performed as staged procedures. Given that, in most cases, AS carries heavier prognostic implication, we believe TAVR should be performed first, with reassessment of MR and symptoms after complete patient recovery prior to the decision to undertake TMVR.
Management Strategy for Patients with Severe AS and Significant MR
Management strategies in patients with concomitant severe AS and significant MR should be based on a thorough clinical and anatomical assessment where age, risk profile, patient preferences, MR severity, and potential for regression after isolated SAVR or TAVR are evaluated. Current guidelines recommend the evaluation of patients in specialized heart valve centers when valve intervention is needed so that the risks and benefits of a surgical versus transcatheter procedure can be discussed by a multidisciplinary team.47 Moreover, patients with truly severe MR are underrepresented in the available literature, limiting generalization of data to this subset of patients.
Figure 2 presents a proposed algorithm of treatment for patients with concomitant AS and MR.
Moderate MR does not represent an indication for therapy; we suggest most patients should be evaluated and treated with the most appropriate AS therapy, SAVR or TAVR, according to the risk profile and anatomic specificities. When the surgical option is selected, mitral surgery may be considered when the MR is organic or with a low potential for improvement.
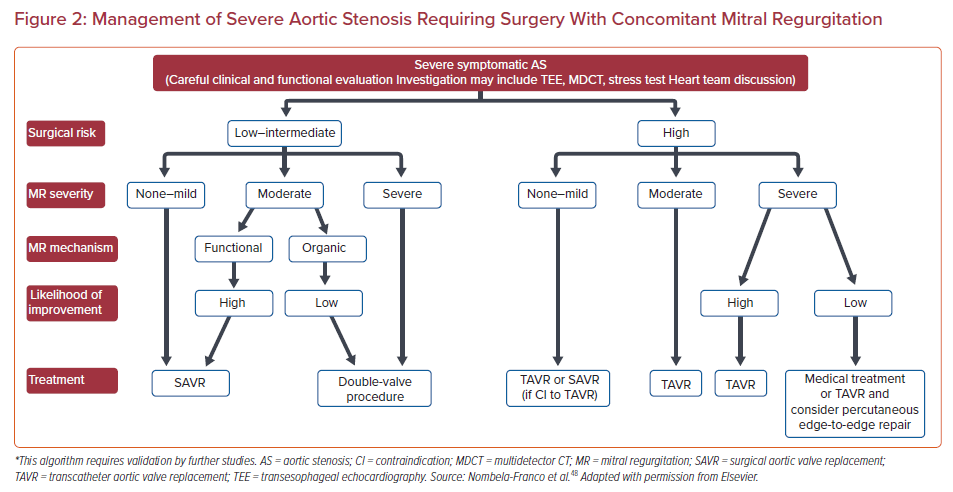
Patients with Grade ≥3 MR are likely to derive benefit from mitral valve surgery when the risk and functional status are appropriate. Low- and intermediate-risk patients with reasonable life expectancy should be considered for double-valve surgery. In our experience, high-risk patients with severe MR represent the more challenging cases for the heart team and have the worse prognosis, regardless of treatment allocation. The added risk of double-valve surgery seems to negate its benefits. In this population of patients, TAVR may become the most reasonable option, more so when the potential for MR regression is real.
However, the risk profile should not dichotomize patients. Some higher-risk patients, such as those with technical challenges with severe organic MR, may derive long-lasting benefit of mitral operation and should, at the very least, be presented with all the options. At the end of the spectrum, although significant MR should not, by itself, discount high-risk patients from TAVR, the lower likelihood of symptomatic improvement and long-term survival should be considered, along with the clinical and functional status, and some patients will best be managed conservatively. In our opinion, most high-risk, elderly patients with non-valvular functional limitations and severe AS along with Grade ≥3 MR should be treated medically.
Staged percutaneous procedures remain a technically feasible and expensive option that have so far met with modest clinical success. Staged percutaneous procedures are probably best reserved for highly selected, symptomatic patients at high risk for surgery, yet having the potential for significant, long-term improvements in quality of life.
Conclusion
For patients with MVD, assessment should include an extensive clinical examination, a thorough anamnesis, and a comprehensive echocardiographic analysis while taking the effects of the various valve pathologies into account. Risk assessment should be done by a heart team in consideration of patients’ comorbidities and treatment goals (survival and improved quality of life). To date, surgical strategies have been the gold standard and remain so in low- and intermediate-risk patients, yet interventional treatment modalities may offer various advantages for high-risk patients. The decision to address AS and MR at the same time or in staged procedures remains at the discretion of the heart team according to which valve is the most significant. Re-evaluation should then be done of the remaining valve pathologies and the clinical status of the patient.