Figure 1 illustrates the procedural steps for provocative vasospasm. Access for the procedure was traditionally femoral to avoid use of calcium channel blockers (CCB) or nitrates, which can blunt the coronary response to Ach. However, the radial artery can safely be used and the vasoactive radial cocktail can vary depending on local practices (i.e. none, CCB, and/or nitrates), and it should be kept in mind that this may blunt the reaction to Ach. In some protocols, NTG is used with the initial diagnostic angiogram and before measurement of iFR/RFR; this may precede Ach infusion and, again, blunt the response. In some protocols, the sequence of administering Ach precedes adenosine and NTG to avoid any possible effect on the vessels’ reactivity to Ach. The use of a smaller, thin-wall 6 Fr sheath and guide catheter is better to reduce potential spasm; 6 Fr catheters are required for thermodilution methods if also performed.
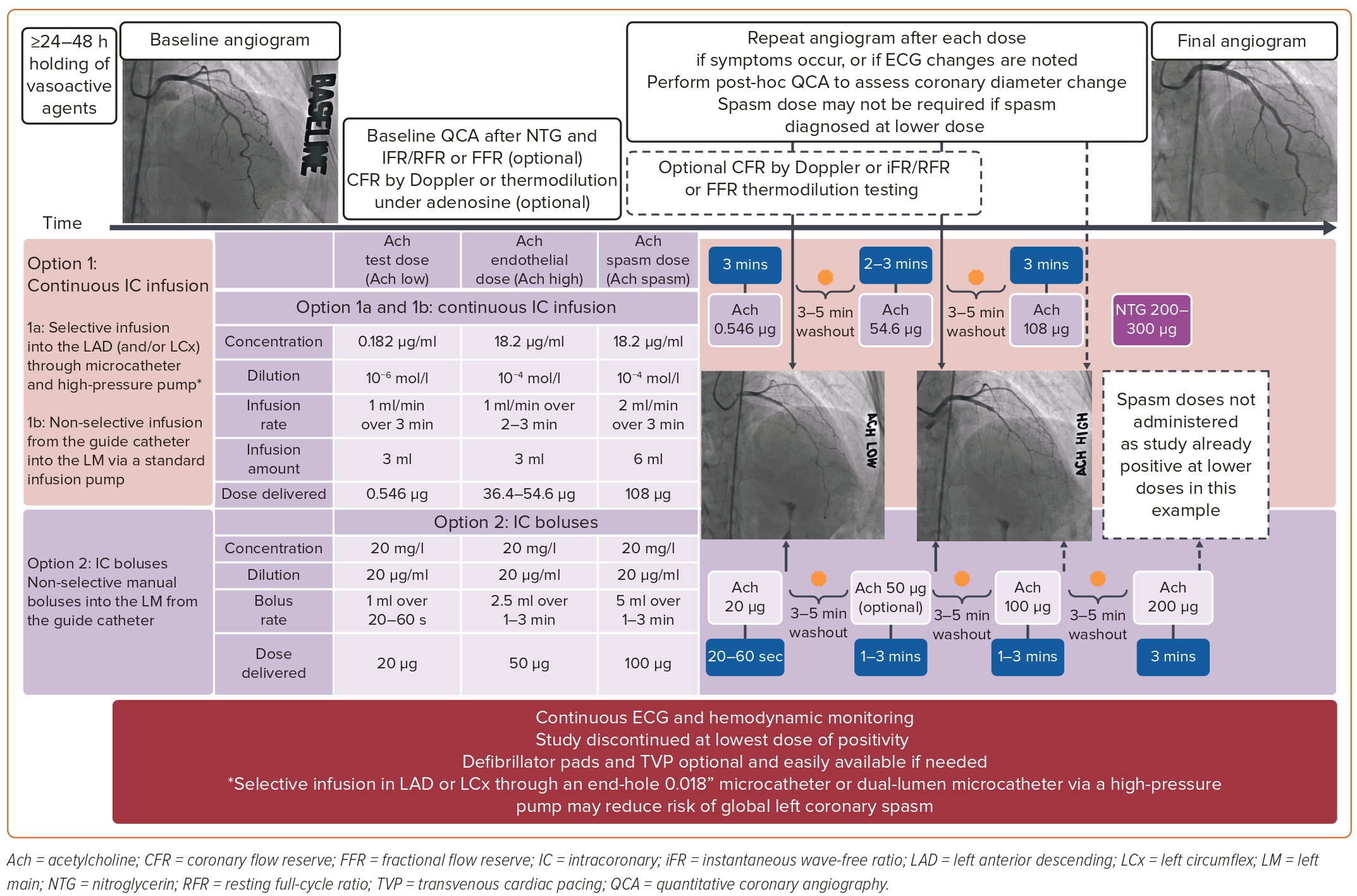
The procedure begins with a baseline diagnostic angiogram of the left coronary artery (LCA) and the RCA, which, in some protocols, is done before any medication administration and in others after NTG; this serves as a reference vessel diameter in a relaxed state.60 A Doppler or thermodilution coronary guidewire is inserted into the coronary artery (which will be used to assess CBF and pressures) and an angiogram is performed to rule out any guidewire-induced or spontaneous vasospasm.
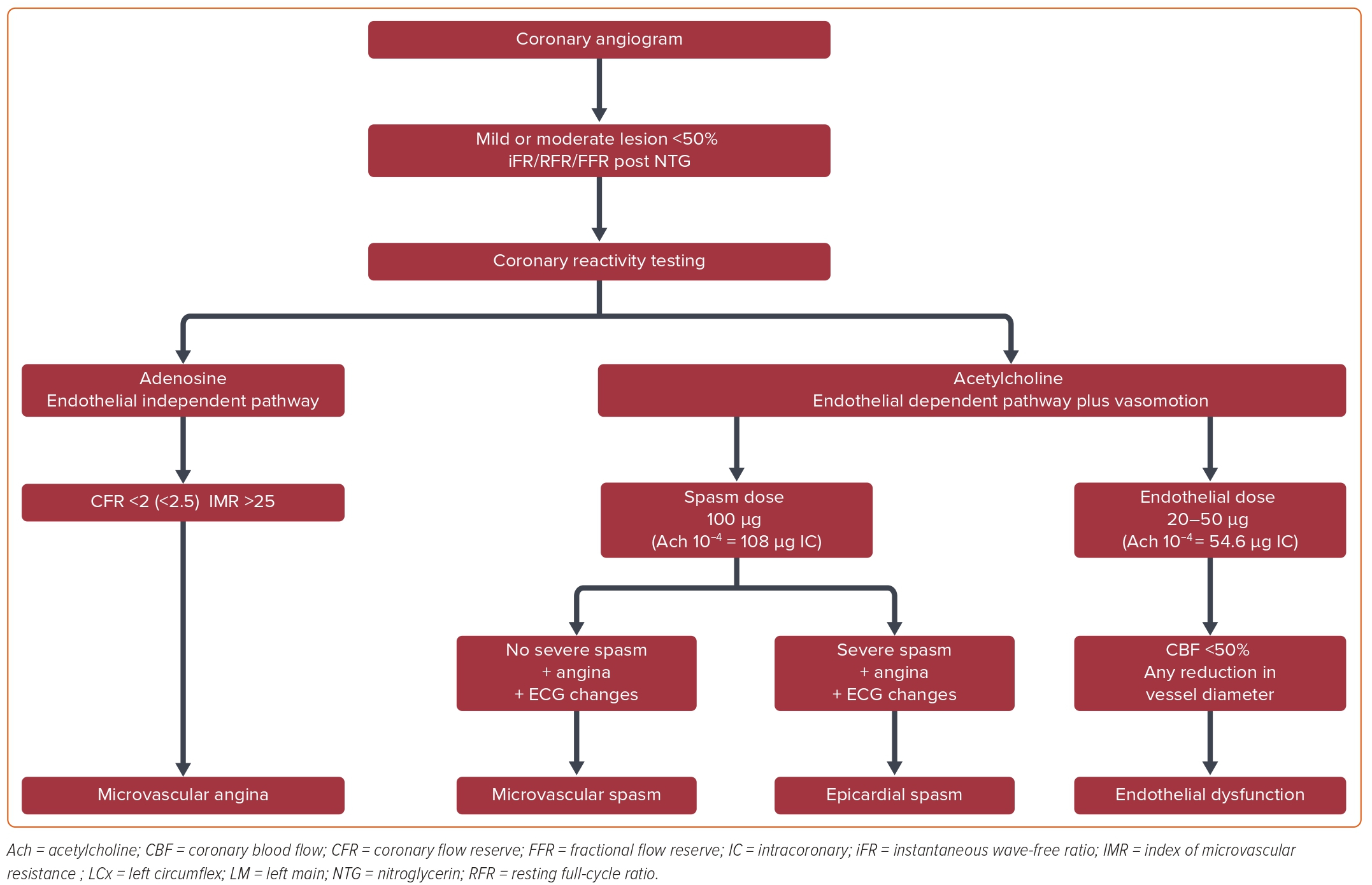
For any moderate to severe epicardial stenoses resting indices, instantaneous wave-free ratio (iFR) and resting full-cycle ratio (RFR) can be performed after NTG without adenosine to rule out hemodynamically significant epicardial disease, which excludes further testing for variant angina.
The measurement of fractional flow reserve (FFR) under IV adenosine is optional, depending on the epicardial stenosis status. In normal coronaries, these measurements are not mandatory. Single-vessel selective injection of the vasoactive provoking agent Ach is performed. The vessel of choice is usually the LAD, which perfuses the biggest portion of the myocardial mass and the corresponding microcirculatory bed. However, the LCx and the RCA can also be used. Single-vessel LAD assessment and analysis leads to a quicker procedure, limits the likelihood of bradycardia that can occur in dominant LCx or RCA systems, and can prevent rare global coronary vasospasms, which in turn can lead to ventricular arrhythmias as a complication. Multivessel analysis may be needed if the initial coronary tests are negative and clinical suspicion remains high.
Injection of provocative agents at different concentrations from low to spasm dose will then follow. An initial test dose of 20 μg of Ach through either a slow injection bolus over 20–60 seconds or via infusion of 0.182 μg/ml at 1 ml/min over 3 minutes for a total of 0.546 μg is given to confirm no severe adverse reaction to Ach. Incremental doses with 3–5 minutes washout periods will follow (Figure 2).11,65,66
A maximum dose of 200 μg through slow bolus can safely be administered without affecting the specificity; however, this higher dose is predominantly used in men when the suspicion of CAS is high and spasm is not provoked by the traditional spasm dose of 100–108 μg, and no greater than 80 μg should be administered in the RCA. However, a recent meta-analysis did not show any major difference in safety or positivity rate from maximum doses of Ach of 100 μg versus 200 μg.67
Figure 2 also displays commonly used protocols with different Ach doses, administered either through a microcatheter directly into the coronary or via boluses from the guide catheter.
A study will be considered positive if a transient, total, or sub-total occlusion of a coronary artery with signs/symptoms of myocardial ischemia (anginal pain and ischemic ST changes) is provoked or is spontaneously observed without any provocation.62,68 When it occurs at any given dosage, a diagnosis of spastic angina can be made and no further escalation of the dose is required.
Lastly, regardless of the provocation medication administered, NTG is injected afterwards and repeat angiography is performed to confirm reversal of the spasm and coronary reactivity to NTG.62
Throughout the study, the patient is monitored for angina symptoms and with continuous 12-lead ECG and hemodynamics.
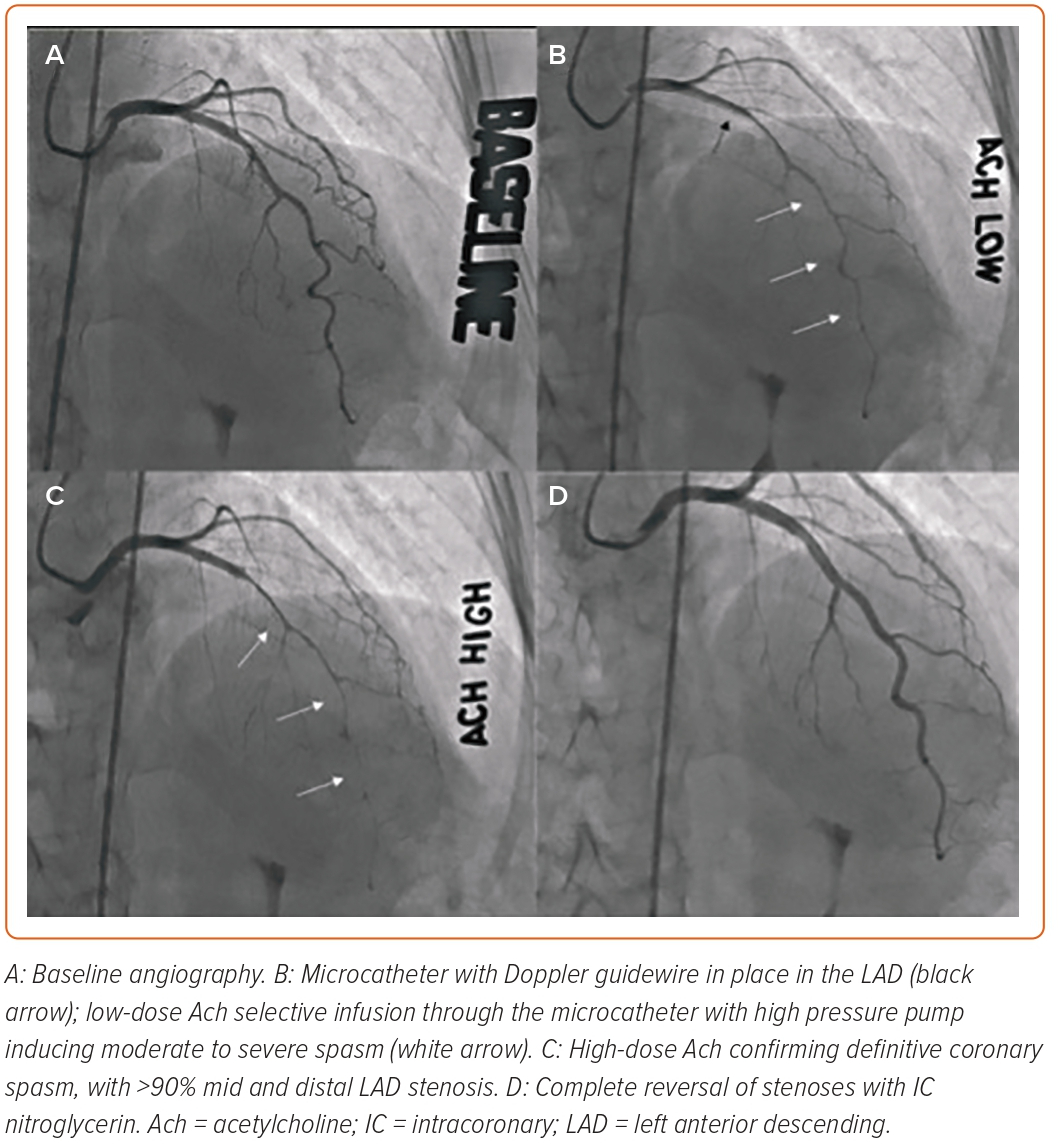
Repeat angiography will be performed in the same projection as the baseline, without catheter or side branch overlap. When vasospasm occurs, pictures are obtained at any dosage of Ach. With spasm, the vessel narrowing can be focal and localized in the main artery or in a branch of a large coronary artery, or be more diffuse from the proximal to the distal segment. Figure 3 demonstrates an example of diffuse LAD vasospasm.
Safety and Adverse Events
Despite a class 2a recommendation from the most recent American Heart Association/American College of Cardiology 2021 chest pain guidelines in patients suspected of INOCA, routine adoption of provocation testing has been limited, possibly due to safety concerns on top of limited availability.69
Additionally, IC Ach (the only provoking agent available in the US) remains off label due to the absence of safety studies, which adds to providers’ apprehension. Ach used in the US is derived from intraocular agents diluted for IC use.
In the current era of selective coronary testing, several studies have reported low event rates, with the most recent meta-analysis from Takahashi et al. confirming a 0% mortality rate with 0.5% of patients presenting major complications, mostly reversible ventricular arrhythmias. The most common side-effects included hypotension, bradycardia, and transient paroxysmal AF, all of which collectively occurred <0.5% of the time and were mainly reversible. Events were more common with RCA compared with LCA testing, supporting current practice to focus on the LCA or selectively the LAD for testing.67,70,71
Sex Differences in Testing
Though the prevalence of CAS has been shown to be greater in women than men, a small retrospective study of Japanese patients by Sueda et al. demonstrated that male patients had significantly higher positive testing rates with Ach compared with ER (80.6% versus 60.6%).72 In the same study, more striking findings were noted in women, with a greater Ach sensitivity over ER (96.7% versus 32.8%). Overall, Ach-provoked spasms occurred more frequently both in men and women and this is therefore believed to be a superior testing method.
Other studies have shown that women may be more sensitive at lower doses of Ach compared to men.73,74 Additionally, diffuse coronary spasms seem to be more prevalent over focal vasospasms in women with Ach provocation. This is notable because diffuse spasms have been shown to portend a better prognosis over focal ones.75
Lastly, sex differences in coronary function testing regarding epicardial spasms versus MVS have been noted, with one study showing men have more epicardial spasms than MVS compared to women.76
Test Limitations
Patients can have catheter-induced spasms, which can be often observed in RCA proximal segments. Catheter-induced vasospasm usually resolves by catheter removal and NTG injection. This should be carefully assessed by the operator, as false-negative results have been observed and reported in the past.77 One explanation for some false negatives is that the provocation test was performed when the disease activity of the coronary vessels was reduced because of the fluctuating nature of CAS.
Kashima et al. reported that false negative provocation tests were more often observed in patients on CCB at the time of and prior to the provocation test.78 This underlines the importance of withholding medication with vasoactive properties for at least 48 hours before the spasm provocation study.60,61
If radial spasm occurs, administering a dose of 100 μg NTG and waiting for 15–30 minutes before starting the provocation procedure may be acceptable. Furthermore, using an Ach dose of 200 μg if spasm is not seen with the 100 μg dose, especially in patients for whom a high suspicion of CAS remains after the 100 μg dose, is advocated.67
Impact of Positive Test on Management and Prognosis
A positive diagnosis carries a prognostic value of future increased cardiovascular events.9 Several treatment strategies have been evaluated, some of which can help mitigate these negative outcomes.
Lifestyle changes and avoidance of precipitating agents are of utmost importance, as many patients are unable to tolerate medical therapy because of side-effects. Therefore, smoking cessation and avoidance of drugs that potentiate coronary vasoconstriction, such as cocaine and sympathomimetic agents, are especially important in patients with VSA.79,80
Furthermore, exercise and a healthy lifestyle will assure overall cardiovascular health. Statins and angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers have been recommended in most patients with ED if they can tolerate them, such as those with hypertension. Ishii et al. studied the effects of statin therapy on patients with VSA and found it was correlated with a lower rate of cardiovascular events in VSA without significant coronary atherosclerotic stenosis.81
More specific antianginal therapies such as CCB and nitrates remain the mainstays of therapy for VSA.82 CCBs dilate VSMCs and have negative inotropic and chronotropic effects.83 Most recently, they were studied in the EDIT-CMD trial, which showed no improvement in CFR but a reduction in CAS in patients with VSA who were on diltiazem 360 mg versus those on placebo.84 A greater proportion of patients on diltiazem progressed from having epicardial spasm to MVS or no spasms at all compared to those on placebo (47% versus 6%; p=0.006). This study once again demonstrated that CCBs can alleviate epicardial spasm, without apparent effects on microvascular non-endothelium-specific CMD.
In practice, CCBs can be used in combination with one dihydropyridine agent (such as amlodipine or nifedipine) being used in addition to a non-dihydropyridine (verapamil or diltiazem) to potentiate each other’s effect on VSMC relaxation while avoiding bradycardia. High doses of each agent will often be required to stabilize the disease.
CCBs have also shown to be beneficial when used in combination with a statin.85 Yasue et al. randomized 64 patients with coronary spasm induced by intracoronary Ach into fluvastatin plus CCB versus CCB alone groups and found that adding fluvastatin reduced Ach-induced coronary spasm at 6-month follow-up.86 CCBs are therefore the first-line therapy for INOCA attributed to coronary spasm, with improved prognosis, angina relief, and quality of life, and decreased severity of epicardial narrowing in response to provocation testing.
Nitrates help to reduce symptoms, dilate the coronary vasculature and reduce ventricular filling pressures.87 Short-acting nitrates play an important role in the treatment of acute VSA episodes as much as for any acute angina episode for any patient with CAD, and sometimes in the chronic prevention of VSA in combination with CCB.55,65
Although nitrates remain widely used in patients with VSA, long-term use of long-acting nitrates may result in further ED, mediated by oxygen free radicals.88 Long-term use of nitrates is also associated with tolerance and rebound vasoconstriction after drug discontinuation, which may be mediated by desensitization of soluble guanylyl cyclase, increased autocrine levels of endothelin, and vascular superoxide production, all of which increase vasoconstrictor sensitivity.89
As such, in several retrospective, observational cohorts, long-term use of nitrates in patients with vasospastic angina has been associated with higher risks of adverse cardiovascular events.90,91 While nitrate use may simply be a marker of more severe underlying disease, long-acting nitrates should be reserved whenever possible as adjunct therapy in patients with VSA refractory to CCB.
β-blockers should be avoided in clear vasospastic syndromes, as alternative treatments with better evidence exist.92 However, in patients with mixed VSA and CMD, most studies support the use of nebivolol for its vasodilatory effect, which may improve parameters of microvascular function without causing vasospasm. Further studies on nebivolol in VSA are needed.
Conclusion
Coronary vasospasm is an important cause of INOCA and MINOCA. Diagnosis relies mainly on invasive cardiac catheterization with spasm provocation and reactivity testing.
There are multiple protocols for spasm provocation, with IC Ach being the most commonly used agent because of its availability, good safety profile, and ease of use. However, consensus on techniques and dosages for Ach administration are lacking, resulting in significant variability in methods and sometimes approaches to diagnosis.
There is a consensus, however, that treatment should be tailored according to the type of INOCA identified, and should therefore be based on the results of a provocation study that tests both endothelial pathway function with Ach and endothelial-independent function with adenosine to identify CMD.
Treatment of isolated VSA may differ from that for VSA in the setting of concomitant CMD or CAD. Confirming or excluding the diagnosis in this patient population will reassure the patient with non-cardiac chest pain, will give a clear diagnosis to a worried patient who had often been told “nothing was wrong in your heart,” and prevent the blind empirical use of antianginals with side-effects such as hypotension or bradycardia.
In summary, invasive testing for coronary vasospasm is part of a comprehensive assessment of INOCA and MINOCA, with the aims of achieving symptom relief and improving the quality of life and prognosis in these patients. 
Clinical Perspective
- Coronary vasospasm can be evaluated only through invasive studies with reliable accuracy.
- The most commonly used provocation agent in the invasive coronary artery spasm assessment is acetylcholine.
- Multiple protocols for acetylcholine use are available; practical and commonly used protocols are discussed in this paper.
- Unified protocols and results interpretation are paramount to accurate diagnosis and a standardized approach to treatment in vasospastic angina.