Coronary angiography is widely used to diagnose coronary artery disease and to guide percutaneous coronary intervention (PCI). However, 2D projection angiography cannot completely reflect the 3D coronary lumen, with several inherent limitations in evaluating plaque composition, vessel diameter, diffuse reference vessel disease, lesion severity, as well as the result of stent deployment. In the past three decades, intravascular ultrasound (IVUS) has been increasingly used in clinical practice to overcome a number of limitations of coronary angiography by providing more details of coronary anatomy and stent implantation.
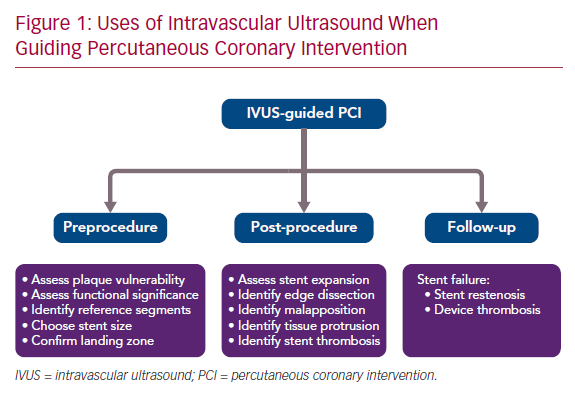
There is growing data from observational studies and randomized controlled trials (RCTs) to validate the value of IVUS guidance in PCI.1–19 IVUS guidance is not routinely performed in PCI, partly due to the increased procedural time, extra cost, and the potential neutral effect on cardiac death. This article summarizes the evidence for IVUS guidance in preprocedural, post-procedural, and follow-up assessment of PCI to highlight the advantage of using IVUS for patients undergoing stent implantation (Figure 1).
Preprocedural Assessment
Basic IVUS Measurement
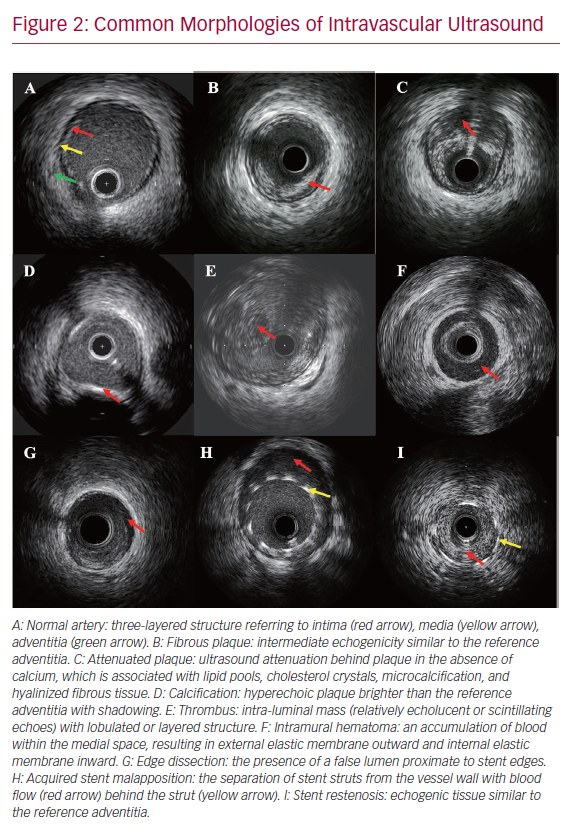
There are three layers in the ultrasound image of coronary arteries (Figure 2).20 The inner layer frequently includes atheroma, intima, and the internal elastic membrane. The middle layer is the media, which is less echogenic than the intima. The outer layer, bordering the external elastic membrane (EEM), consists of the adventitia and periadventitial tissues, which cannot be distinguished from each other in IVUS images. IVUS can be used to make the following basic measurements:
- Minimum lumen diameter (MLD): the shortest diameter through the center point of the lumen.
- Minimum lumen area (MLA): the smallest area through the center point of the lumen.
- Lumen eccentricity: (maximum lumen diameter − minimum lumen diameter)/maximum lumen diameter.
- Area stenosis: (reference lumen area − stenosis lumen area)/reference lumen area.
- Plaque burden: (EEM area − lumen area)/EEM area.20
Assessment of Plaque Vulnerability
Vulnerable plaque, sometimes called thin-cap fibroatheromas (TCFA), often associated with large plaque burden, spotty calcifications, attenuated plaque, and shallow echolucent zones shown by gray-scale IVUS, is a common cause of MI and cardiac death.21–25 In virtual histology (VH)-IVUS, TCFA was defined as focal, necrotic core-rich plaque (≥10% of the cross-sectional area) in contact with the lumen with atheroma volume ≥40%.26,27 The international, multicenter trial An Imaging Study in Patients with Unstable Atherosclerotic Lesions (PROSPECT) enrolled 697 patients with acute coronary syndrome undergoing coronary angiography, gray-scale and radiofrequency IVUS. It showed that three lesion-level independent predictors of major adverse cardiovascular events (MACE) at a median follow-up of 3.4 years were MLA ≤4 mm2 (HR 3.21; 95% CI [1.61–6.42]), plaque burden ≥70% (HR 5.03; 95% CI [2.51–10.11]), and the presence of TCFA (HR 3.35; 95% CI [1.77–6.36]).28 In this study, TCFA was defined as the presence of >10% confluent necrotic core with more than 30% of the necrotic core abutting the lumen in ≥3 consecutive frames by VH-IVUS. Moreover, the VH-IVUS in Vulnerable Atherosclerosis (VIVA) study and the European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis – Intravascular Ultrasound (ATHEROREMO-IVUS) study confirmed the finding that TCFA identified by VH-IVUS was associated with MACE.27,29 However, the PROSPECT trial also showed that the combination of these three high-risk plaque features only had an 18.2% predictive value for MACE, which might be due to the low resolution of IVUS, which makes it difficult to detect plaque composition.28
Assessment of Functional Significance
There is always high inter-observer variability for angiographic estimation of the degree of coronary stenosis.30,31 Intermediate coronary lesions, defined by 40–70% stenosis by angiography assessment, cannot be accurately evaluated for their hemodynamic significance for MI even by experienced interventional cardiologists. Fractional flow reserve (FFR) has been regarded as the gold standard method of invasive MI assessment.32–34 Anatomic data for MLA has a relatively good correlation with FFR, which could be a liberal diagnostic application, though potential errors exist due to the variations of BMI and lesion complexity.
A comprehensive meta-analysis demonstrated that an IVUS-derived MLA of 2.8 mm2 for non-left main coronary lesions with an angiographic diameter >3 mm, and 2.4 mm2 for lesions with a diameter <3 mm, were cut-off values to detect functionally significant coronary stenosis.35 In isolated intermediate left main coronary lesions involving the ostium or shaft, an IVUS-derived MLA of 5.9 mm2 had the highest sensitivity (93%) and specificity (95%) for determining FFR less than 0.75 in Western populations, while an MLA <4.5 mm2 had good correlation with an FFR under 0.80 (77% sensitivity and 82% specificity) in Asian populations.36,37
The multicenter, prospective Spanish Working Group on Interventional Cardiology (LITRO) study also showed that an IVUS-derived MLA of more than 6 mm2 was a safe value to use to defer coronary revascularization of the left main lesions.38 Therefore, it seems reasonable to perform revascularization for left main coronary lesions when there is an MLA of <4.5 mm2, to defer revascularization if there is an MLA of >6 mm2 and to consider further invasive or non-invasive functional evaluation if there is an MLA of 4.5–6 mm2.
IVUS-guided Preprocedural Preparation
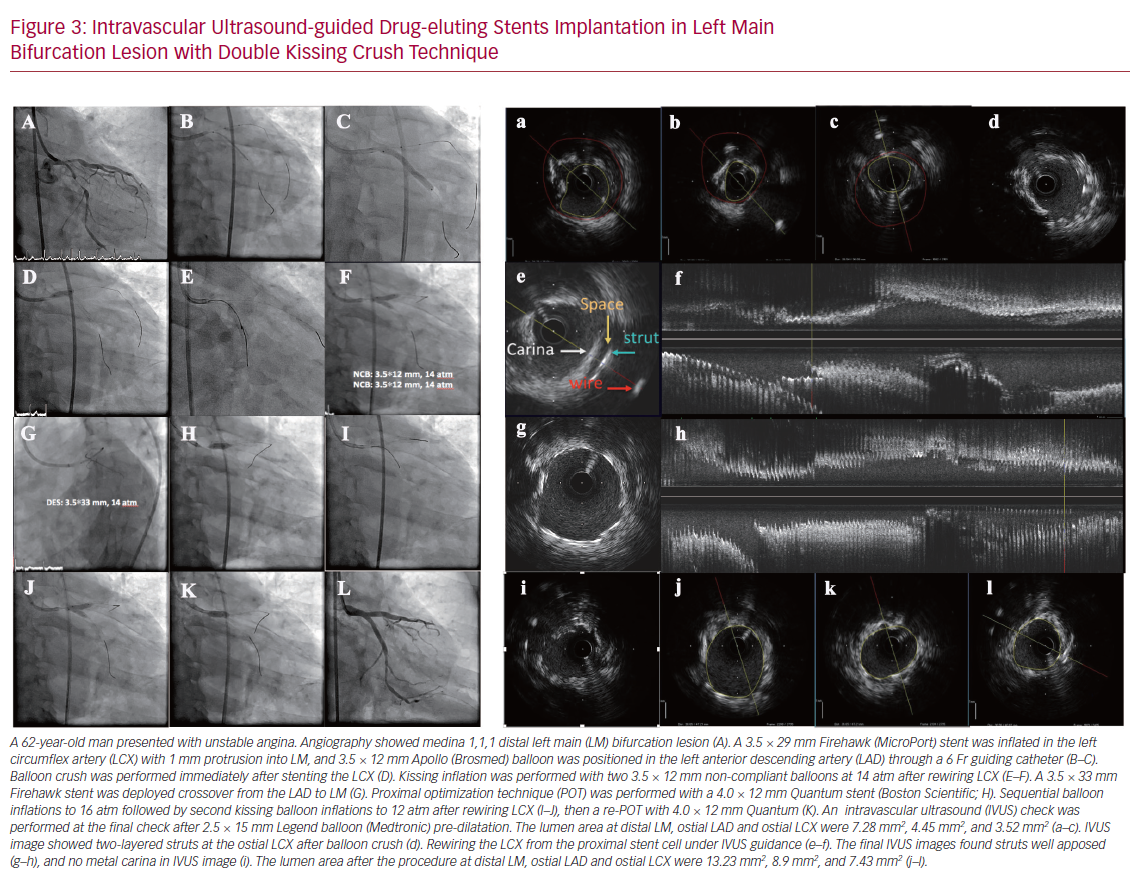
A preprocedural IVUS check is important to assess calcium severity, to select stent size, to identify a reference segment and confirm the landing zone (Figure 3). Angiography is moderately sensitive for the detection of extensive calcific lesions, but it is less sensitive for mild calcium. One study has shown that IVUS could detect calcium in 841 of 1,155 (73%) stable patients, whereas angiography detected calcium in only 440 (38%) of them.39 The presence of severe calcium may require predilatation with a higher inflation pressure, larger balloon, cutting balloon angioplasty, or rotablator atherectomy.
Several potential IVUS-based stent diameter methods exist, including stent diameter according to EEM diameter at the site of MLA, the smallest reference EEM diameter, the largest reference lumen, mean reference lumen, or the smallest reference lumen. Currently, the use of mean diameter of distal lumen with post-dilatation of the proximal and middle part of the stent is recommended.40 Stent length is determined by the distance from the distal to proximal reference site. Proximal and distal reference sites are set at cross-sections adjacent to the target lesion that have the largest lumen and a plaque burden of <50%.41 An appropriate landing zone is commonly considered as having residual plaque burden <50% without lipid-rich plaque at the stent edge, which is associated with subsequent stent restenosis.40–42 Overall, compared with angiography guidance, IVUS guidance could lead to more stents, larger stent diameter, longer stent length, and a greater post-procedural minimum stent area (MSA).12,19,43-45
Post-procedural Assessment
Post-procedural IVUS assessment can detect stent underexpansion, acute stent malapposition, stent deformation, tissue protrusion through the stent struts, stent edge dissection, and residual disease at stent edge (Figures 2 and 3). Several studies have demonstrated that stent underexpansion was associated with early stent thrombosis and restenosis after implantation of a drug-eluting stent (DES).46–49 Post-procedural MSA has been considered the most important parameter to predict these adverse events.46–51 The current view is that the optimal MSA measured by IVUS is >5.5 mm2 for non-left main lesions, >7 mm2 for distal left main lesions, and >8 mm2 for proximal left main lesions.40,47,48,52,53 Acute stent malapposition after DES implantation without underexpansion does not translate into early or long-term adverse events regardless of the length and thickness of malapposition.54–56
An IVUS subgroup analysis from the Assessment of Dual AntiPlatelet Therapy With Drug Eluting Stents (ADAPT-DES) trial showed that 34.3% of lesions presented with tissue protrusion detected by IVUS after DES implantation. This was not associated with worse 2-year clinical outcomes, in part due to the larger lumen area of lesions treated with larger stent or post-dilatation balloon in the tissue protrusion group.57
However, a substudy of the Bivalirudin in Patients Undergoing Primary Angioplasty for Acute Myocardial Infarction (HORIZONS-AMI) trial demonstrated that significant tissue protrusion (plaque/thrombus), defined as residual lumen area <4mm2 by IVUS detection, was associated with early stent thrombosis.46 Moreover, post-procedural large edge dissections – characterized by IVUS as deep depth (at least disrupting the media layer) great lateral extension (>60°C), and long length (>2 mm) – could result in early stent thrombosis.46,58
The optimal criteria of IVUS-guided bare-metal stent deployment in the Multicenter Ultrasound Stenting in Coronaries Study (MUSIC) included:
- complete apposition of stent;
- adequate stent expansion: MSA ≥90% of average reference lumen area or ≥100% of the smaller reference segment area if the MSA <9 mm2, or MSA ≥80% of average reference lumen area or ≥90% of the smaller reference segment area if the MSA >9 mm2; and
- symmetrical stent expansion: MLD/maximum lumen diameter ≥0.7.
The MUSIC study found that 81% of 155 patients undergoing bare metal Palmaz-Schatz stents met IVUS optimal criteria, and the overall risk of target lesion revascularization was 4.5% at 6 months.59
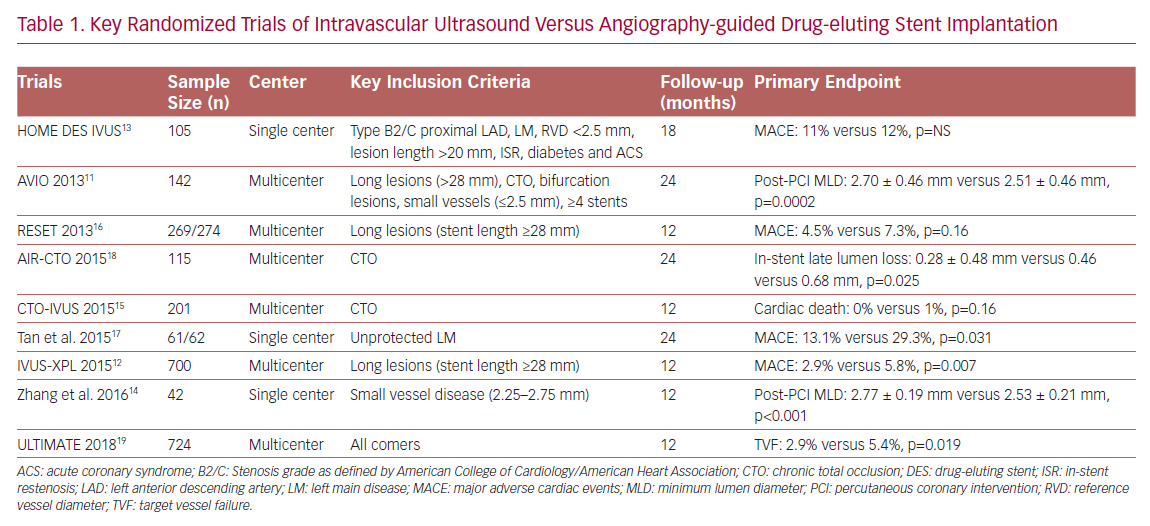
In the DES era, evidence derived from observational studies, RCTs (Table 1) and meta-analyses all demonstrated that IVUS-guided DES implantation was associated with a lower risk of MACE and target vessel revascularization (TVR) in complex lesions, such as unprotected left main disease, bifurcation lesions, chronic total occlusion, and long lesions.1–5,8,10–18,43,44,60–65 More importantly, several optimal IVUS-guided criteria have been proposed through RCTs rather than observational studies. The Angiography Versus (vs) IVUS Optimisation (AVIO) RCT, proposed a new optimal IVUS-guided criterion: MSA >70% of the post-dilatation balloon cross-sectional area (CSA), and the non-compliant post-dilatation balloon size to be determined by the average of the media-to-media diameters of distal in-stent segment, proximal in-stent segment, and maximal in-stent narrowing.11
Another randomized trial – the Impact of Intravascular Ultrasound Guidance on Outcomes of Xience Prime Stents in Long Lesions (IVUS-XPL) study – presented an IVUS-guided optimal criterion for long lesions as the MLA greater than the lumen CSA at distal reference segments.12,65 Most RCTs have enrolled people with complex coronary lesions, and only the Intravascular Ultrasound Guided Drug Eluting Stents Implantation in ‘All-Comers’ Coronary Lesions (ULTIMATE) trial recruited all-comer patients and showed that IVUS guidance was associated with significant lower risk of target vessel failure (TVF) compared with angiography guidance in all-comers undergoing second-generation DES implantation.19 In this trial, the novel criteria of IVUS-guided optimal DES deployment were:
- the MLA in the stented segment >5.0 mm2, or 90% of the MLA at the distal reference segments;
- plaque burden of 5 mm proximal or distal to the stent edge <50%; and
- no edge dissection involving media with a length >3 mm.
A total of 53% of the 1,448 participants met these three criteria and they were associated with a lower rate of TVF at 12 months compared with those with a suboptimal PCI procedure. An updated meta-analysis that included the ULTIMATE trial demonstrated that IVUS guidance could reduce the risk of cardiac death.66 A 5-year clinical follow-up of the IVUS-XPL trial has shown the long-term benefit of IVUS guidance in optimizing DES implantation in long lesions.65 However, further RCTs are warranted to explore the difference in clinical relevance when using different optimal IVUS guidance criteria.67
Follow-up Assessment
IVUS at follow-up is used to detect chronic stent expansion, stent fracture, neointimal hyperplasia, stent malapposition, and positive remodeling of vessel wall. Current guidelines and expert consensus recommend intracoronary imaging should be used to identify the mechanisms of stent failure (restenosis and thrombosis) at follow-up.32,40,68
The common causes of stent restenosis, apart from intimal hyperplasia, are chronic underexpansion, stent fracture, and neoatherosclerosis.47,69 Chronic underexpansion and stent fracture could be assessed easily by IVUS, but the detection of neoatherosclerosis may need a higher resolution intracoronary modality. A prospective, multicenter study showed that stent fracture could be found in 803 (12.3%) patients, 3,630 (22.0%) stents, and 1,852 (17.2%) diseased vessels, which was associated with higher risk of stent restenosis and definite stent thrombosis.70 In this study, a novel classification of stent fracture was proposed, identifying five types:
- type IA: single strut fracture;
- type IB: gap between 2 struts >2 times a 2.5mm cell;
- type II: incomplete transverse, V gap;
- type III: complete transverse, no displacement; and
- type IV: complete transverse with displacement.
Of 3,630 fractured stents in this study 2,963 were detected by angiography and the remaining 640 had to be identified by IVUS. Stent malapposition at follow-up should be divided into two types: persistent malapposition since stent implantation and late acquired malapposition, which may be caused by plaque/thrombus resolution and positive remodeling.54
Conclusion
IVUS can provide important information about vessel lumen, dimensions, plaque characteristics, and stent deployment, as well as the mechanisms of device failure. Clinical studies have demonstrated that IVUS-guided PCI could improve the clinical outcomes in patients with DES implantation, especially for complex coronary lesions and high-risk patients. But IVUS guidance is not routinely performed in the real-world daily practice of PCI, partly due to the increased procedural time and extra cost. The next step should be to reduce the cost of IVUS, educate interventional cardiologists, and promote the use of IVUS as much as possible during PCI.