“When you fail to prepare, you’re preparing to fail.”
– Coach John Wooden
Ongoing technological and procedural innovations increasingly permit the safe and effective performance of ever more high-risk and complex percutaneous coronary interventions (PCI) in the modern cardiac catheterization laboratory. Anatomic characteristics of higher risk include unprotected distal left main stenosis, bifurcation and trifurcation lesions, chronic total occlusions, vein graft lesions, last remaining vessels supplying large myocardial territories, and heavily calcified lesions.1–3 Moderate-to-severe coronary calcification is encountered in up to one-third of coronary lesions, is commonly associated with a greater degree of lesion complexity to include coronary bifurcations and chronic total occlusions, and is expected to increase in prevalence with increasing patient age, chronic kidney disease, and diabetes.4–6
Patients with severely calcific coronary lesions have overall higher mortality, worse outcomes independent of clinical presentation, and are less likely to receive complete revascularization.2,7,8 Heavily calcified lesions are difficult to dilatate, and are more commonly associated with coronary dissection and perforation, failure to deliver stents, stent underexpansion and malapposition, polymer disruption and impaired drug delivery with drug-eluting stents, and higher rates of target lesion failure, restenosis, stent thrombosis, MI, and death.2,4,9–11
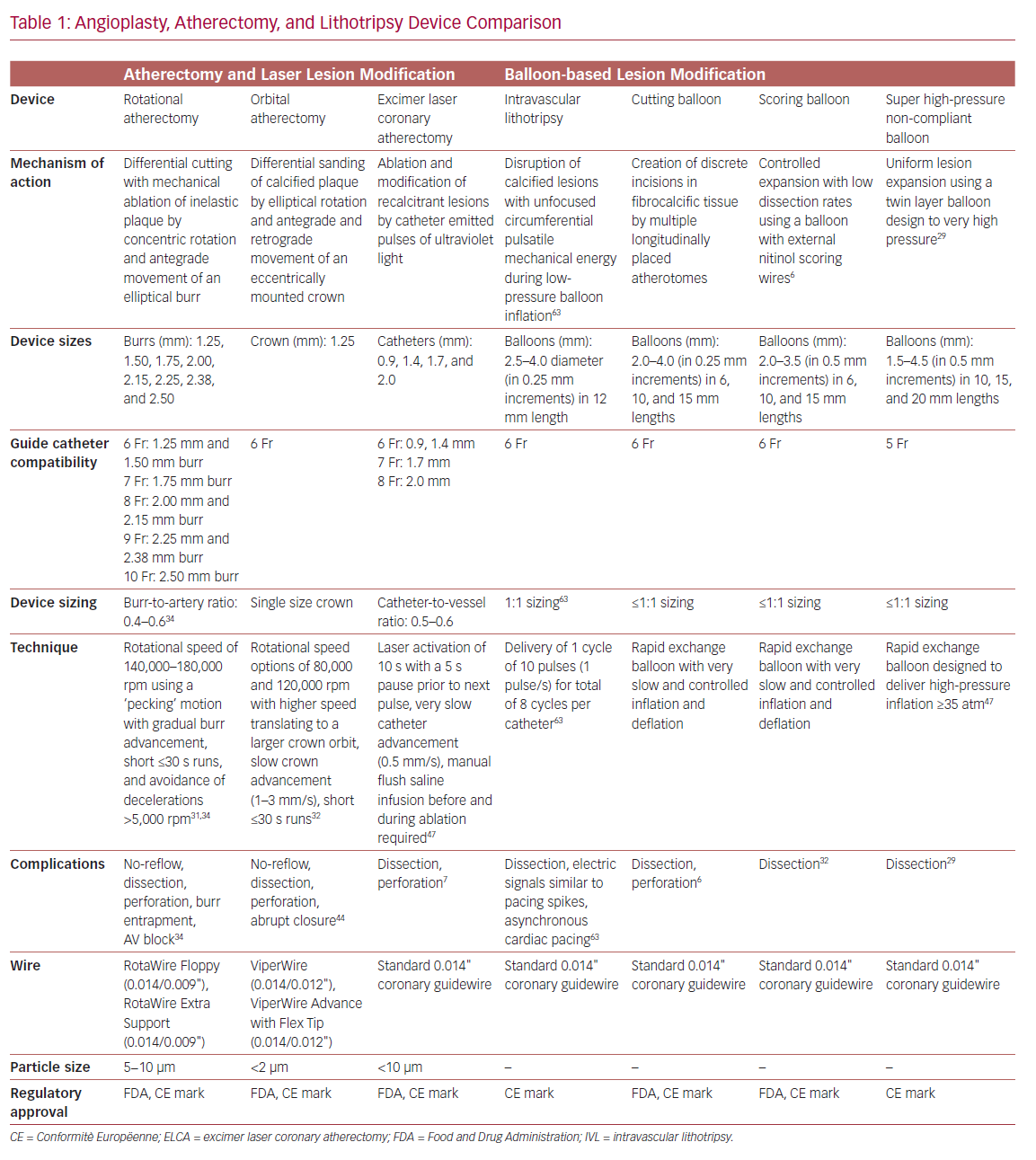
Technologies to include high-pressure non-compliant balloons, ultrahigh-pressure balloons, cutting balloons, and various forms of atherectomy are all designed to facilitate PCI in severely calcified coronary arteries (Table 1). Over time, atherectomy has evolved from a stand-alone debulking therapy to a bail-out technique in undilatatable stenoses or an adjunct to balloon angioplasty, and finally into a primary approach of intentional lesion preparation.12–15 The contemporary objective of atherectomy is plaque modification to disrupt the continuity of calcium, and enable balloon inflation and stent implantation and expansion while also minimizing side branch compromise.16 Up-front versus bail-out atherectomy is also associated with decreased fluoroscopy dose, shorter procedural times, lower contrast volume, and the use of fewer predilatation balloons.17 Despite such advantages, atherectomy is employed infrequently in the US, and its use remains highly variable across operators and hospitals.1
Lesion Assessment
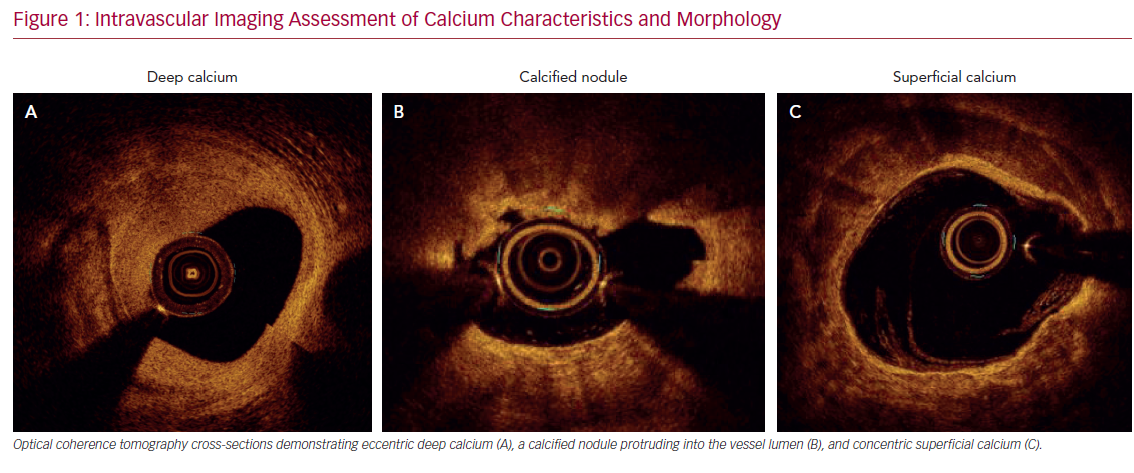
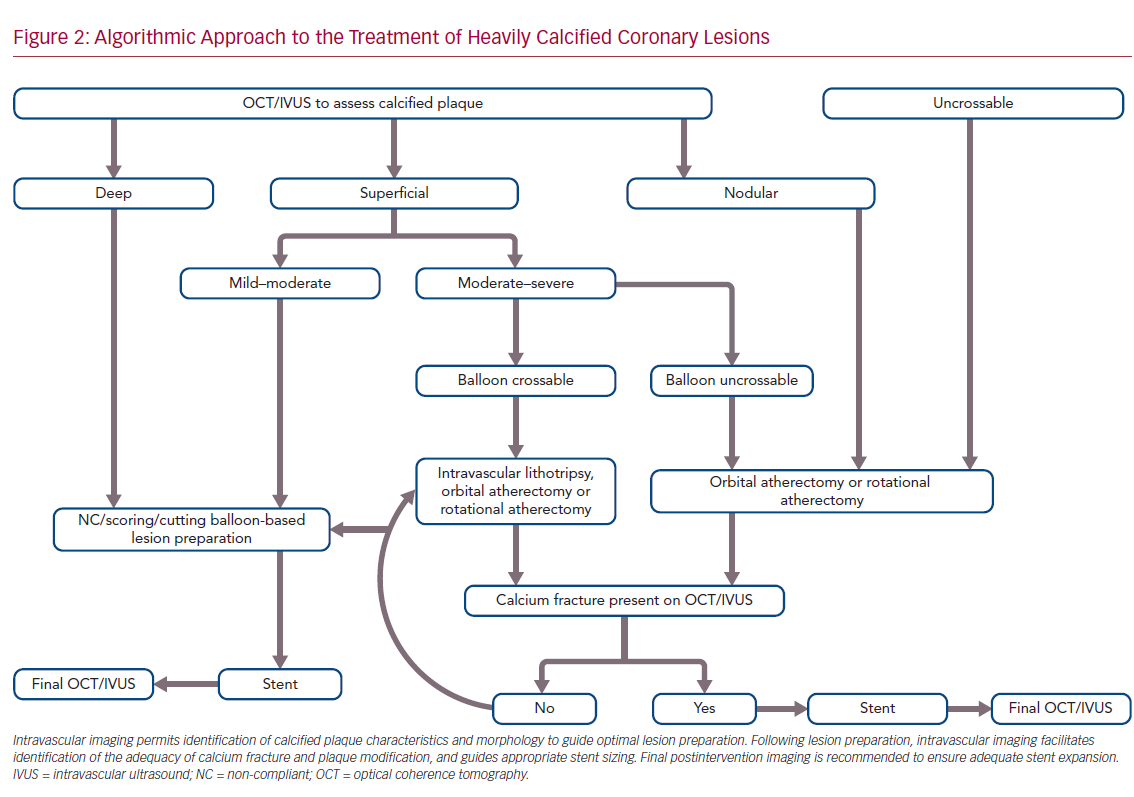
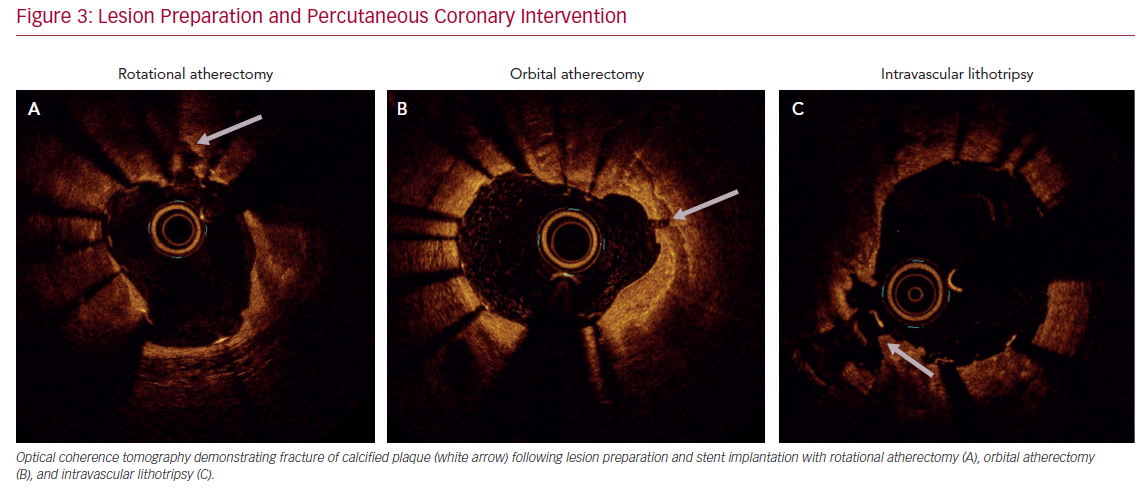
Accurate characterization of calcium distribution and morphology is key to successful treatment. Coronary angiography grossly underestimates the presence, extent, severity, and depth of coronary calcification (Figure 1).18,19 Advanced intravascular imaging techniques enhance identification of calcium, and permit comprehensive assessment of calcium burden, eccentricity, depth, distribution, and the presence or absence of a calcified nodule, thereby facilitating a tailored strategy for lesion preparation and appropriate atherectomy device selection (Figure 2).20 Intravascular imaging simplifies complex decision-making, reduces radiation and contrast exposure, provides accurate measurement of luminal size, lesion length, and reference vessel diameter, offers visualization of projected stent landing zones, and ensures that adequate postprocedure endpoints, to include stent expansion and apposition, and the absence of edge dissections, have been achieved (Figure 3).21 As is the case with atherectomy, despite an association with improved clinical outcomes, the use of intravascular imaging remains low in the US, with substantial interoperator and interhospital variability.22,23
Intravascular ultrasound (IVUS) is the most reliable diagnostic tool to detect coronary calcium with high tissue penetration. However, due to acoustic shadowing, IVUS reveals the calcific arc without defining its thickness. Despite more limited depth penetration, optical coherence tomography, with its higher spatial (axial and longitudinal) resolution, offers more accurate quantification of calcific plaque, such as calcium area, thickness, length, and 3D volume, all of which better predict response to balloon dilatation.24–26 Optical coherence tomography also has higher sensitivity compared with IVUS in detecting both stent malapposition and underexpansion.27
Balloon Angioplasty
Balloon-based techniques do not remove calcium, but aim to increase plaque elasticity and allow stent expansion by cracking calcified zones in one or multiple areas. Standard balloon dilatation of calcified lesions often results in non-uniform balloon expansion and consequent overexpansion of, and injury to, more compliant vessel segments. Non-compliant balloons apply higher inflation forces and facilitate more uniform balloon expansion. The newer OPN non-compliant balloon (SIS Medical) permits uniform expansion up to super high pressures of 40 atm, but may still be biased toward non-calcified vessel segments leading to dissection at the fibrocalcific interface.28,29 Cutting balloons utilize multiple atherotomes placed longitudinally on the balloon surface to concentrate dilatation forces along the blades to create radial incisions with more controlled intimal and medial dissections.30 However, they are often too bulky to cross undilatable lesions and may further fail to expand lesions with significant calcification, even at high pressures.
Rotational Atherectomy
Rotational atherectomy (RA; Boston Scientific) employs a diamond-coated elliptical burr to rotate concentrically while advancing in a forward direction. Differential cutting permits mechanical ablation of inelastic fibrocalcific plaque while sparing adjacent compliant elastic vessel tissue.31,32 The effect of RA on severely calcified lesions depends on the calcium eccentricity, luminal area, burr size, and degree of guidewire bias.33 When circumferential calcium is present and the minimal luminal area is smaller than the burr size, RA effectively drills a circular lumen inside the calcium.34 When the burr used is smaller than the luminal area, calcium modification is only seen in areas where guidewire bias directs the burr toward the vessel wall.
Most operators use standard workhorse coronary wires for lesion crossing followed by microcatheter wire exchange, as both versions of the RotaWire are limited by poor lesion crossability. The Floppy RotaWire is most commonly used for RA, although the Extra Support wire may preferentially be employed to alter wire bias and ablate plaque at the lesser curvature (versus greater curvature) of angulated lesions, or for aorto-ostial or distal vessel lesions.35 Cutting balloons may sometimes also be deployed after RA to facilitate more complete calcium fracture and greater stent expansion.36
Contemporary best practices, to include appropriate lesion selection, proper burr-sizing, optimal atherectomy speed and duration, integration of visual, tactile, and auditory feedback and strategies to anticipate, prevent and manage adverse events are well reviewed elsewhere.31,34 Procedural complications, such as no-reflow, and vessel dissection and perforation, are largely preventable with optimal technique. Transient conduction disturbances may likewise be prevented by shorter RA runs, longer breaks between runs, and prophylactic pharmacological measures, thereby avoiding the risks associated with temporary pacemaker implantation.37
Orbital Atherectomy
Orbital atherectomy (OA; Cardiovascular Systems) has a crown design with diamond chips on both the front and back to allow ablation during both antegrade and retrograde motion while facilitating continuous blood flow during both rest and ablation, thereby reducing thermal injury, transient heart block, no reflow, and the risks of crown entrapment.38–41 OA utilizes differential sanding during fast elliptical rotation of the crown using centrifugal force to ablate hard non-compliant calcium while sparing normal healthy vascular tissue, and demonstrates deeper tissue ablation compared with RA.42 Increased orbit diameter and deeper ablation arcs are created using a single-sized crown by decreasing advancement speed and increasing contact time between crown and plaque, increasing the number of passes, increasing rotation speed, and altering wire bias.43,44
Laser Atherectomy
Excimer laser coronary atherectomy (ELCA; Philips) modifies tissue by three distinct mechanisms: photochemical, photothermal, and photomechanical effects.45,46 Laser interaction with calcium occurs primarily via the photomechanical component, and is increased if laser is applied directly through blood (without saline) and even more so if used with contrast injection.33,47 Excimer laser catheters are compatible with standard coronary guidewires, and may also be used to create a pilot channel to permit subsequent microcatheter passage for follow-on RA (“RASER” technique) or OA to leverage the combined benefits of each individual technology.48–50
Intravascular Lithotripsy
The intravascular lithotripsy (IVL) system (Shockwave Medical) is a novel balloon catheter-based device that utilizes pulsatile mechanical energy to disrupt calcified lesions. Miniature emitters placed along the length of a semicompliant balloon convert electrical energy into transient acoustic circumferential pressure pulses that disrupt both superficial and deep calcium within vascular plaque.51,52 The balloon is typically sized 1:1 to the reference vessel diameter, and inflated to subnominal pressures to permit contact with the vessel wall while minimizing static barotrauma.
IVL uniquely modifies calcium both circumferentially and transmurally, and has a preferential effect on deep calcium compared with other ablation techniques.51 In contrast to both RA and OA, which generate nanoparticles that may embolize distally and impair microcirculatory function, larger calcium fragments generated by lithotripsy remain in situ. As a balloon-based technique, it is also user-friendly with a short learning curve. Balloon uncrossable lesions remain the primary limitation of IVL, and sometimes balloon predilatation or alternative atherectomy (such as RA, OA, or ELCA) may be required to facilitate balloon delivery.
Special Situations
PCI in heavily calcified lesions may be performed safely via radial access with standard use of 7.0–8.5 Fr sheathless guide catheters.53 Adjunctive devices and techniques to include guide extension catheters, anchor balloon techniques, and specialty wires may be required for equipment delivery for all angioplasty, atherectomy, and lithotripsy devices.54,55
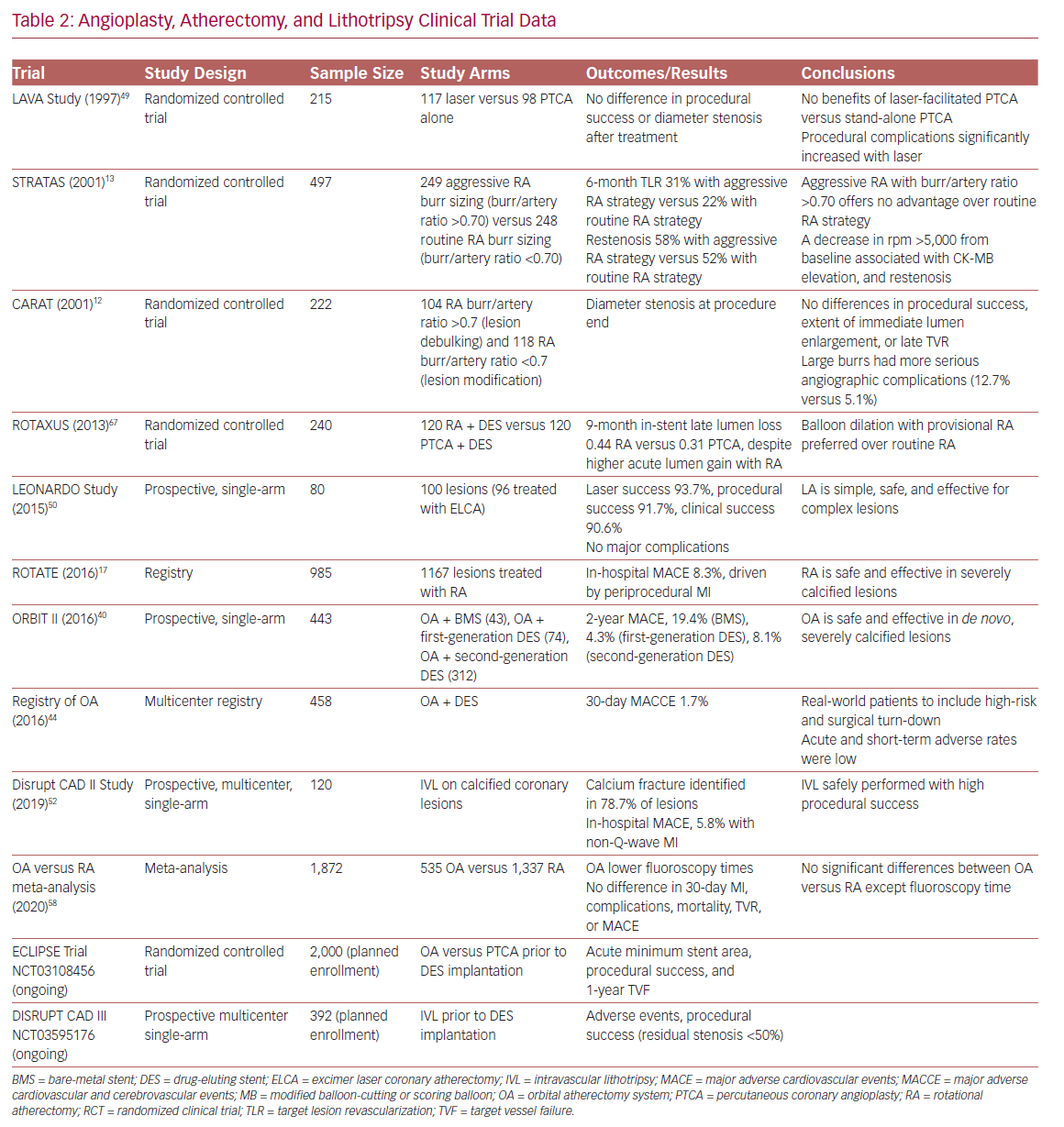
There are no randomized controlled trials directly comparing different atherectomy technologies (Table 2). Device choice should consider patient and angiographic characteristics, local availability, and operator comfort and institutional expertise. Although there may exist specific advantages in certain lesion subtypes and clinical scenarios, the principal objective is to ensure adequate lesion preparation regardless of the device utilized.56–58 New mobile apps, such as CalcificAid (https://cardiologyapps.com/calcificaid), are also now available to guide device selection and procedural techniques.
OA has the potential advantage of ease of use and compatibility of a single-size device with a 6 Fr guide or 7 Fr guide/extension combination. RA has higher penetration power, as the device ablative interface sits immediately at the burr tip (whereas the OA ablative crown is located 6.5 mm proximal to the tip), and may therefore have theoretical advantages in ostial lesions, balloon uncrossable lesions, and chronic total occlusions (even in the presence of dissections).34,59 ELCA may be uniquely used for chronic total occlusion cap modification by expert operators.45 IVL has the distinct advantage of permitting plaque modification without surrendering wire position, which may be especially attractive in bifurcation lesions or lesions with high risk of side branch compromise. Finally, in instances of underexpanded stents due to failure to adequately perform lesion preparation in heavy concentric calcification during the index procedure, RA (‘stentablation’), OA, ELCA with contrast medium (‘laser bomb’), or IVL may all be utilized.60–64
Future Directions
Intravascular imaging and lesion preparation therapies remain underused technologies in the face of progressively increasing patient and lesion complexity. Several ongoing randomized clinical trials evaluating the most appropriate and cost-effective use of various intravascular imaging, angioplasty, atherectomy, and lithotripsy technologies are currently underway, including Evaluation of Treatment Strategies for Severe CaLcIfic Coronary Arteries: Orbital Atherectomy versus Conventional Angioplasty Technique Prior to Implantation of Drug-Eluting StEnts (ECLIPSE; NCT03108456) and Disrupt Coronary Artery Disease (DISRUPT CAD III; NCT03595176). Future efforts should target consensus imaging guidelines and operator training pathways to guide the best selection of appropriate therapies, and ensure adequate stent implantation, and optimal short- and long-term outcomes in patients with heavy vessel calcification.3,25,65,66
Conclusion
Coronary artery calcification continues to increase in prevalence in parallel with an aging patient population, and rising rates of diabetes and renal disease. PCI of increasingly complex calcified coronary artery lesions remains a persistent challenge in contemporary interventional practice. Growing use of intravascular imaging to inform a strategic treatment algorithm is proving critical to optimal patient management. Newer device technologies increasingly facilitate more complete and effective revascularization with superior short-term procedural results and long-term patient outcomes.