Although there have been significant advances in therapies to prevent and treat cardiovascular disease, cardiovascular events remain the most common cause of morbidity and mortality. In the US, cardiovascular disease has a mortality rate higher than all types of cancer and pulmonary disease combined.1 Coronary artery disease accounts for approximately 40% of deaths from cardiovascular disease.1 Acute coronary syndromes (ACS), which can be broken down into unstable angina (UA), non-ST-elevation MI (NSTEMI), and ST-elevation MI (STEMI), account for many of these events. An MI will occur every 40 seconds, with more than 700,000 of the general population in the US having a new coronary event and another 300,000 having a recurrent event.1 This article focuses on the optimal pharmacotherapy for patients who present with ACS and the patient populations these strategies may be targeted to based on clinical trials and guidelines.
Pathophysiology
ACS occur when a lipid-rich thin atherosclerotic plaque ruptures.2–4 This exposes the endothelium to thrombogenic substances, such as platelets and clotting factors, which leads to thrombus formation, limiting blood flow through the coronary vasculature. Platelets bind to the exposed endothelial surface while also activating other platelets via thrombin, thromboxane A2, adenosine diphosphate (ADP), epinephrine, and other mediators.2–4 Platelet binding occurs via glycoprotein (GP) IIb/IIIa receptors expressed on platelet membranes, cross-linking activated platelets to other platelets via fibrinogen. Activated monocytes release metalloproteinases and tissue factor, which interact with the vulnerable plaque.2–4 Activation of these different pathways results in platelet aggregation and activation of the clotting cascade, leading to thrombus formation. If the vessel is partially occluded, UA or NSTEMI may occur, whereas complete occlusion may lead to STEMI. The degree of obstruction and the timing of presentation influence the degree of myocardial damage.
Anti-ischemic Agents
As patients may present with ischemic chest pain, initial therapy may be with nitrates.5,6 Nitrates work by increasing guanosine 3′5′-monophosphate in smooth muscle, forming nitric oxide, which promotes systemic and coronary vasodilatation and increases collateral flow to ischemic areas.5,6 Preload is also decreased, relieving symptoms of congestion and volume overload. These actions help improve myocardial blood flow supply and limit oxygen demand, relieving symptoms but having no effect on mortality. Sublingual nitroglycerin can be administered as three doses, if needed, given 5 minutes apart. If symptoms are not relieved, IV nitroglycerin may be initiated and titrated until chest pain resolves. If there is right ventricular involvement or recent use of sildenafil, nitrates should be avoided for at least 24 hours (and for at least 48 hours after the use of tadalafil) because significant hypotension can occur.7 Chronic nitrate usage leads to tolerance, which is alleviated by either increasing the dose, which may be necessary in ACS, or allowing for a nitrate-free period of 10–12 hours.
Ranolazine is an alternative to nitroglycerin for chronic angina and works by inhibiting late sodium channel influx during myocardial repolarization, thereby reducing intracellular calcium concentrations, which leads to lower ventricular wall tension and decreased myocardial oxygen consumption.8 The anti-ischemic effects of ranolazine are achieved without changing blood pressure or heart rate. Ranolazine is dosed at 500–1,000 mg twice daily and has a half-life of 7 hours. The MERLIN-TIMI 36 trial found no difference in major adverse cardiovascular event (MACE) outcomes with ranolazine in ACS compared with placebo.9 However, recurrent ischemia was significantly reduced in those randomized to ranolazine (13.9%) compared with placebo (16.1%; p=0.03).9 Because hemodynamics are not changed by ranolazine, it is an option for those patients already on maximal anti-anginal therapy whose blood pressure and heart rate are controlled.
Antiplatelet Agents
Aspirin
Aspirin irreversibly binds to cyclooxygenase-1 on platelets, inhibiting the production of thromboxane A2, a potent stimulus of platelet aggregation. In the setting of ACS, 325 mg of regular aspirin should be administered because it is absorbed rapidly and attains platelet inhibition within 1 hour after ingestion.10 After a 325 mg loading dose, aspirin should be maintained at daily dose of 75–100 mg because higher doses do not provide any benefit but increase the risk of bleeding.11 Although aspirin has a plasma half-life of approximately 20 minutes, the effect of aspirin lasts approximately 7 days due to its irreversible binding to platelets. Aspirin prevents future vascular events and has similar effects when compared with fibrinolytics administered without aspirin.12 Aspirin, along with thienopyridines, is required in patients undergoing percutaneous coronary intervention (PCI) because stent placement damages the endothelium, making that territory vulnerable to thrombosis. However, as described below, aspirin may not be necessary for patients who require more intense antithrombotic therapy.
Thienopyridines
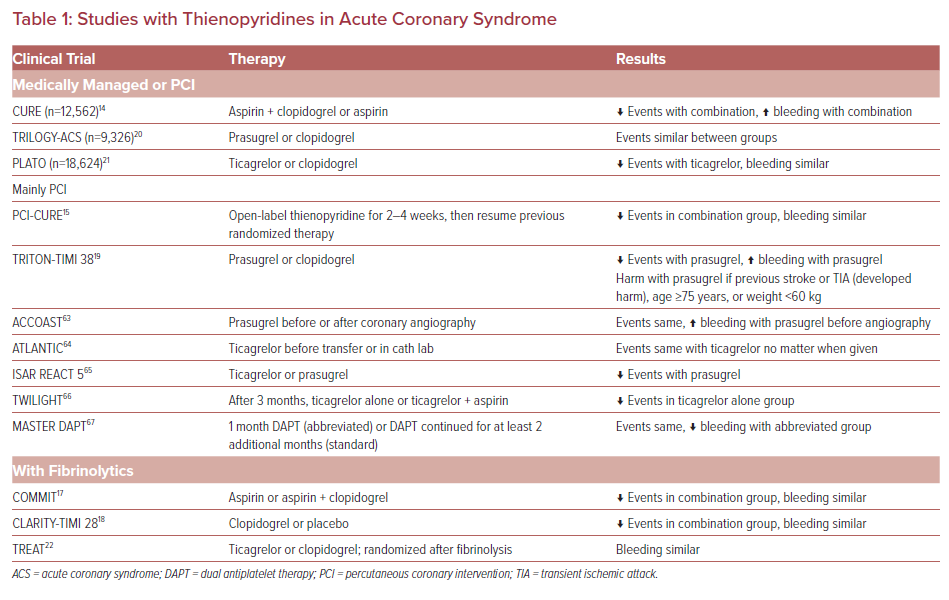
Thienopyridines bind to ADP P2Y12 receptors on platelets, preventing platelet aggregation, and a summary of major trials with these agents is provided in Table 1. Clopidogrel is a prodrug that requires a two-step conversion process to its active metabolite.13 Cytochrome P450 family 2 subfamily C member 19 (CYP2C19) is the main enzyme involved in the formation of the active metabolite of clopidogrel. This metabolite has a half-life of approximately 30 minutes and is rapidly removed from the circulation unless it binds to the P2Y12 receptor.13 Clopidogrel irreversibly binds to the P2Y12 receptor and has a duration of action of 5–7 days. A loading dose of 300 mg clopidogrel inhibits platelets at 8–12 hours, but with 600 mg clopidogrel platelets are inhibited within 2 hours.13 Some limitations of clopidogrel include its delayed onset of action, the need for its conversion to the active form, and its prolonged offset. Based on currently available data, in the setting of ACS that is managed with PCI or medically with fibrinolysis, clopidogrel has been shown to decrease the risk of MACE with a slight increase in major bleeding.14–18
Prasugrel was the next thienopyridine available after clopidogrel. Prasugrel is also a prodrug, but requires only one step to be converted to its active metabolite, primarily through isoenzymes cytochrome P450 family 3 subfamily A member 4 (CYP3A4) and cytochrome P450 family 2 subfamily B member 6 (CYP2B6), and has a slightly longer half-life (60 min) than clopidogrel.13 Prasugrel is more potent than clopidogrel, with higher plasma concentrations of the active metabolite reached after the administration of lower doses of prasugrel.13 Prasugrel also irreversibly binds to platelets and has a duration of action of 7–10 days. In the setting of ACS, prasugrel is best used in patients after undergoing coronary angiography and who have had their coronary anatomy defined because it has benefits in reducing MACE, especially stent thrombosis, but at the cost of an increased risk in major bleeding; it has no benefits in patients who are medically managed alone.19,20 Three patient subgroups that do not benefit from prasugrel are those with a history of stroke or transient ischemic attack (TIA), those aged ≥75 years, and those with a body weight <60 kg. Patients with a history of stroke or TIA had MACE and bleeding event rates that trended higher with prasugrel such that use of prasugrel is an absolute contraindication in this population.19
Ticagrelor is a non-thienopyridine cyclopentyltriazolopyrimidine agent that reversibly binds to P2Y12 receptors.13 Because ticagrelor binding to the P2Y12 receptor is reversible, restoration of platelet function occurs 3–5 days after administration. Although an active metabolite is formed when the parent compound is metabolized by CYP3A4/5 enzymes, ticagrelor does not require hepatic metabolism to an active form for platelet inhibition.13 The half-life of ticagrelor is 6–12 hours and, because of its reversible binding, it is administered twice daily. The shorter duration of action and not requiring metabolism to an active form provide advantages for ticagrelor use in ACS compared with clopidogrel and prasugrel. In addition, similar to clopidogrel, ticagrelor use resulted in decreased MACE among patients who were medically managed.21 Limited information is available in patients administered fibrinolytics, but the available data suggest that ticagrelor may be administered after the fibrinolytic is given, with similar bleeding events to those seen with clopidogrel.22
Cangrelor is the only IV P2Y12 inhibitor available, and it binds to these platelet receptors reversibly.23,24 Like ticagrelor, cangrelor does not require conversion to an active metabolite. Dosing in the setting of PCI is a 30 µg/kg bolus followed by a 4 µg/kg/min infusion for the duration of the PCI procedure. The half-life of cangrelor is approximately 3 minutes, and platelet function returns to normal within 1 hour of stopping the infusion.23,24 Cangrelor is approved in the setting of PCI in patients who have not already been treated with a P2Y12 inhibitor, so its use may be limited to STEMI patients who are not able to take oral medications or in settings of UA/NSTEMI where having a short-acting agent may be beneficial (e.g. after recent major surgery). The CHAMPION PHOENIX trial evaluated the use of cangrelor versus clopidogrel in patients requiring PCI in the setting of ACS.25 The rates of MI and stent thrombosis were lower with cangrelor, but there was a slight increase in the risks of major and minor bleeding.25 One of the limitations of cangrelor use is that trials have only compared it to clopidogrel, and not more potent thienopyridines, and the process of switching to other oral thienopyridines has not been investigated. Because cangrelor binds the P2Y12 receptor, its infusion must be discontinued before clopidogrel or prasugrel can be administered.24 However, because ticagrelor binds to a different site on the P2Y12 receptor, it can be given during cangrelor infusion or immediately after cangrelor infusion is discontinued.
Glycoprotein IIb/IIIa Receptor Antagonists
Activated platelets express the GP IIb/IIIa receptor, allowing platelets to cross-link to each other through fibrinogen, the final common pathway of platelet aggregation.26,27 GP IIb/IIIa inhibitors work by blocking the receptor from binding to fibrinogen, preventing platelet cross-linking.26,27 During the early use of PCI, GP IIb/IIIa inhibitors were widely used to prevent post-procedural complications such as vessel thrombosis and MI. Currently, there are two commercially available GP IIb/IIIa inhibitors, eptifibatide and tirofiban (production of a third, abciximab, ceased as of 2019). Both eptifibatide and tirofiban bind to the GP IIb/IIIa receptor in a reversible manner and provide >80% inhibition when used at recommended doses. Both agents are also cleared through the kidneys, requiring dose adjustments in patients with decreased renal function.
As studies in ACS and PCI with thienopyridines have been performed, the use of GP IIb/IIIa inhibitors has become limited due to similar MACE event rates with less bleeding. The EARLY-ACS trial randomized 9,492 patients presenting with non-STEMI ACS to eptifibatide or placebo.28 Patients were assigned either to an early, routine administration of eptifibatide or placebo with delayed, provisional administration of eptifibatide after angiography but before undergoing PCI. There was no significant difference in the occurrence of the primary composite endpoint of death from any cause, MI, recurrent ischemia requiring urgent revascularization, or thrombotic bailout at 96 hours between the early use of eptifibatide and the delayed, provisional administration of eptifibatide (9.3% versus 10%, respectively; p=0.23). However, thrombolysis in MI (TIMI) major bleeding was significantly increased with the early versus delayed administration of eptifibatide (2.6% versus 1.8%, respectively; p=0.02).28 In contemporary practice, because of no benefit but an increased risk of bleeding, the use of GP IIb/IIIa inhibitors should be restricted to those patients with a significant thrombus burden or as bailout when coronary flow is significantly reduced.
Antithrombotic Agents
Along with platelet activation, the clotting cascade is activated after plaque rupture. Anticoagulation is necessary in the setting of both fibrinolysis (to prevent re-occlusion of the newly patent vessel) and PCI (to prevent thrombus formation from intravascular devices used during the procedure, as well as the damage that may occur following balloon angioplasty and stent placement). Numerous studies have examined different anticoagulants in this setting of ACS, with heparin and bivalirudin being the agents most commonly used.
Heparin is a heterogeneous mixture of polysaccharide chains of varying lengths with a large molecular weight.29,30 Heparin works by potentiating the effects of antithrombin, an endogenous anticoagulant that mainly inhibits Factor IIa (thrombin) and Factor Xa via the irreversible inhibition of activation sites on these factors. The benefits of heparin include its widespread availability, the ability to monitor its effects, its short half-life, its reversal with protamine, and decreased cost. The limitations of heparin include its need for antithrombin as a cofactor, its variable anticoagulant effect, and the risk of causing heparin-induced thrombocytopenia.
Bivalirudin is a small synthetic peptide that is an analog of hirudin.30 Bivalirudin works by reversibly and directly binding to thrombin. The benefits of bivalirudin compared with heparin include its ability to bind to fibrin-bound thrombin, predictable anticoagulant effects, and a slightly shorter half-life (25 minutes versus 90–120 minutes).30 The limitations of bivalirudin include the lack of an antidote and its cost compared with unfractionated heparin.
Initial studies comparing heparin to bivalirudin showed that bivalirudin decreased rates of major bleeding in patients but increased the rate of acute stent thrombosis. In the HEAT-PPCI open-label single-center trial, patients scheduled for primary PCI were randomly assigned to heparin or bivalirudin to investigate the incidence of MACE and major bleeding at 28 days.31 In all, 1,829 patients were randomized, with nearly all patients loaded with aspirin and a thienopyridine and approximately 80% of patients having the procedure completed with radial access. MACE rates were lower in the heparin than bivalirudin group (5.7% versus 8.7%, respectively; p=0.01). New MI or reinfarction and stent thrombosis were also lower in the heparin group. Major bleeding was similar in both groups (3.1% versus 3.5% in the heparin and bivalirudin groups, respectively; p=0.59).31 These findings were confirmed in the VALIDATE-SWEDEHEART trial, which compared bivalirudin versus heparin in ACS patients receiving a potent thienopyridine.32 In that study, the bivalirudin and heparin groups had similar rates of both MACE (7.2% versus 8%, respectively; p=0.21) and major bleeding (5.1% versus 5.6%, respectively; p=0.32).32 Based on the introduction of more potent antiplatelet therapy, increased use of radial access, and similar MACE and bleeding outcomes, using heparin in the setting of PCI is a more cost-effective strategy than using bivalirudin.
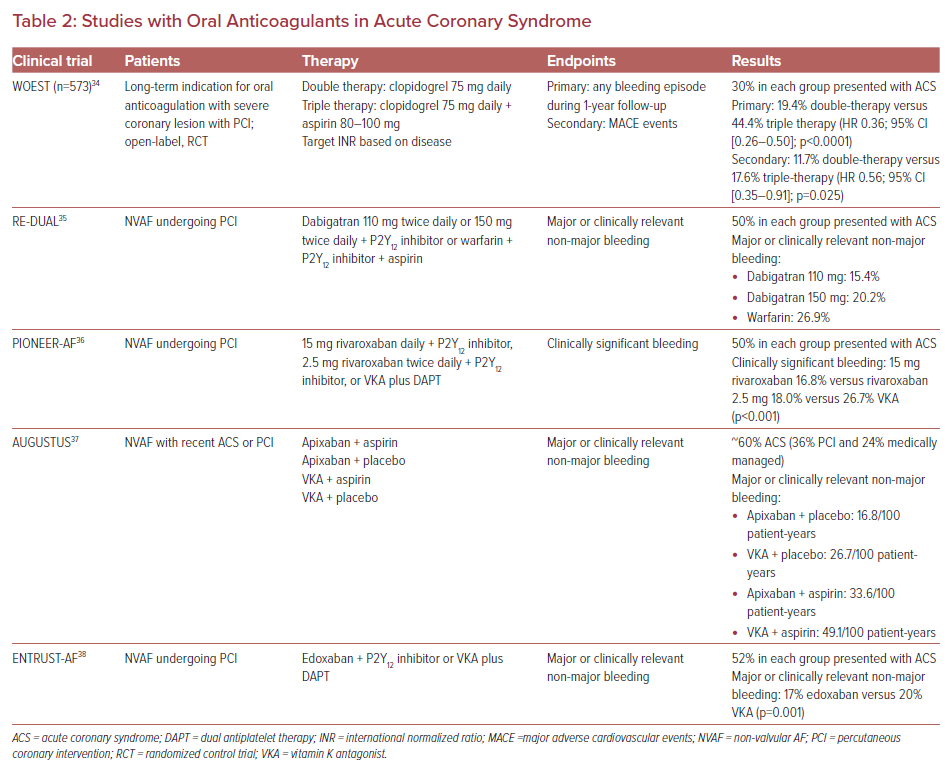
Up to 10% of patients requiring PCI also have an indication for therapeutic anticoagulation, mostly for non-valvular AF.33 Bleeding risk with dual antiplatelet therapy and an anticoagulant ranges from 10% to 30%, depending on the definition. Strategies to decrease this risk include only using a thienopyridine and a vitamin K antagonist or using a direct oral anticoagulant instead of a vitamin K antagonist. The WOEST trial was the first to investigate these strategies, showing that a less antithrombotic strategy may be used to decrease bleeding events.34 However, that study was small, did not include many ACS patients, and only included vitamin K antagonists for anticoagulation. Direct oral anticoagulants have been shown to have similar efficacy and a better safety profile compared to vitamin K antagonists in patients with non-valvular AF. The oral agents available are the direct thrombin inhibitor dabigatran and the Factor Xa inhibitors apixaban, edoxaban, and rivaroxaban. Compared with warfarin, these agents have an earlier onset of action, a more predictable anticoagulant response, and fewer drug interactions. All these agents have some degree of renal clearance, which should be taken into account for dosing and patient selection. Recent studies that have included ACS patients who require long-term anticoagulation following PCI are summarized in Table 2.35–38 Based on the current evidence, the best strategy for ACS patients requiring anticoagulation for non-valvular AF following PCI is to use clopidogrel as the thienopyridine, to limit the duration of aspirin use or to avoid using aspirin following the procedure, and to use a direct oral anticoagulant for long-term anticoagulation. Clinicians should be aware that these trials mainly focused on bleeding outcomes and were not powered to evaluate MACE events.
Fibrinolysis
Not all patients who present with STEMI will have ready access to PCI, especially those in developing countries and those who live remotely to PCI centers. Early administration of fibrinolytics is indicated in these patients to open the occluded artery, abate symptoms, and prevent further cardiovascular complications. Fibrinolytics convert plasminogen to plasmin, which breaks down fibrin within the thrombus, leading to clot dissolution.39 Fibrinolytics should be administered as early as possible, preferably within 1 hour of symptom onset, and at least within 12 hours of symptoms.39 Patients who receive fibrinolytics should be transferred to a PCI facility in case lysis fails, as indicated by the failure of ischemic symptoms to resolve with ST-segment elevation or no resolution of ST-segment elevation. If there are no contraindications, these patients should have immediate coronary angiography and rescue PCI if indicated. Absolute contraindications for fibrinolytics are active internal bleeding, intracranial neoplasm or surgery within the previous 2 months, uncontrolled hypertension, and bleeding diathesis. The three fibrin-specific agents available in the US are alteplase, reteplase, and tenecteplase. Alteplase has high fibrin specificity and is given as a 15 mg bolus, followed by 0.75 mg/kg infused over 30 minutes and finally 0.5 mg/kg infused over 60 minutes, with the maximum dose being 100 mg. Reteplase has moderate fibrin specificity and is given as two 10-unit boluses, each given over 2 minutes, 30 minutes apart. Tenecteplase has very high fibrin specificity and is administered as a weight-based single bolus ranging from 30 mg (if weight <60 kg) up to 50 mg (if weight ≥90 kg).
Lipid-lowering Therapy
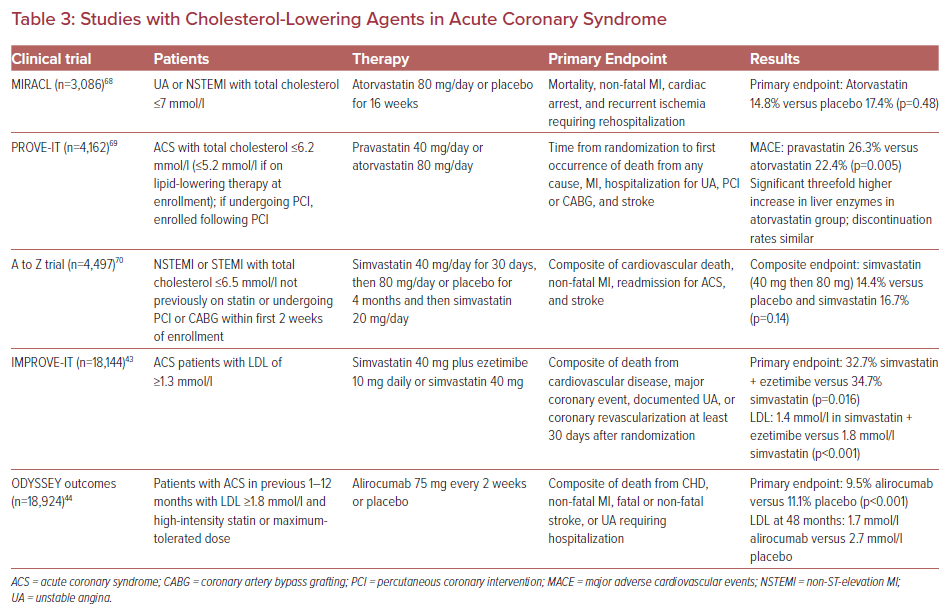
The use of aggressive lipid-lowering therapy for patients presenting with ACS would fall into the category of established CHD. Initial therapy should use 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) inhibitors, also known as statins. Inhibition of HMG-CoA leads to a reduction in cholesterol synthesis, as well as upregulation of hepatic LDL receptors, which further promotes clearance of LDL.40 Statins and other lipid-lowering agents have been shown to decrease future atherosclerotic events, especially when used with especially high-intensity statin therapy, as described in Table 3. High-intensity statins result in a 50–60% decrease in LDL. In addition to lowering LDL, statins have numerous pleiotropic effects, such as improving endothelial function, stabilizing atherosclerotic plaques, reducing fibrinogen, and anti-inflammatory effects.41 Patients should be targeted to an LDL level of <1.8 mmol/l. Baseline liver function tests are recommended prior to the initiation of therapy, but routine monitoring is no longer recommended because severe hepatic injury is very rare.
For those not able to achieve an LDL target of <1.8 mmol/l with high-intensity statins, the addition of a non-statin lipid-lowering therapy is recommended. Ezetimibe works at the brush border of the small intestine, inhibiting the Niemann-Pick C1-Like 1 (NPC1L1) transporter.42 Cholesterol levels are reduced because inhibition of the NPC1L1 transporter leads to reduced delivery of cholesterol to the liver and subsequent upregulation of LDL receptors, allowing for increased LDL uptake by the liver.42 Ezetimibe has a half-life of 22 hours, is dosed at 10 mg daily, and is generally well tolerated. When ezetimibe is added to statin therapy, an additional decrease in LDL of approximately 24% is expected. In ACS, the addition of ezetimibe to simvastatin in IMPROVE-IT led to a decrease in cardiovascular events, mainly because of a reduction in MI.43
Some patients may not achieve targets with statin or statin plus ezetimibe therapy. In addition, some patients may not tolerate statins due to adverse effects such as myopathy. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been recently introduced as lipid-lowering agents with a different mechanism of cholesterol lowering to existing agents. PCSK9 inhibitors work by binding to PCSK9, preventing the breakdown and increasing the number of LDL receptors.40 When PCSK9 inhibitors are added to statin therapy, an additional decrease in LDL of approximately 60% is expected. In a recent trial with alirocumab in patients with a recent ACS already on high-intensity statins or a maximally tolerated dose of statin, the addition of PCSK9 inhibitor therapy decreased MACE and achieved a mean LDL level of 1.7 mmol/l at 48 months.43 These data suggest that the addition of alirocumab to patients already on statin therapy provides further reductions in cardiovascular events compared with statins alone.
Renin–Angiotensin–Aldosterone System Inhibition
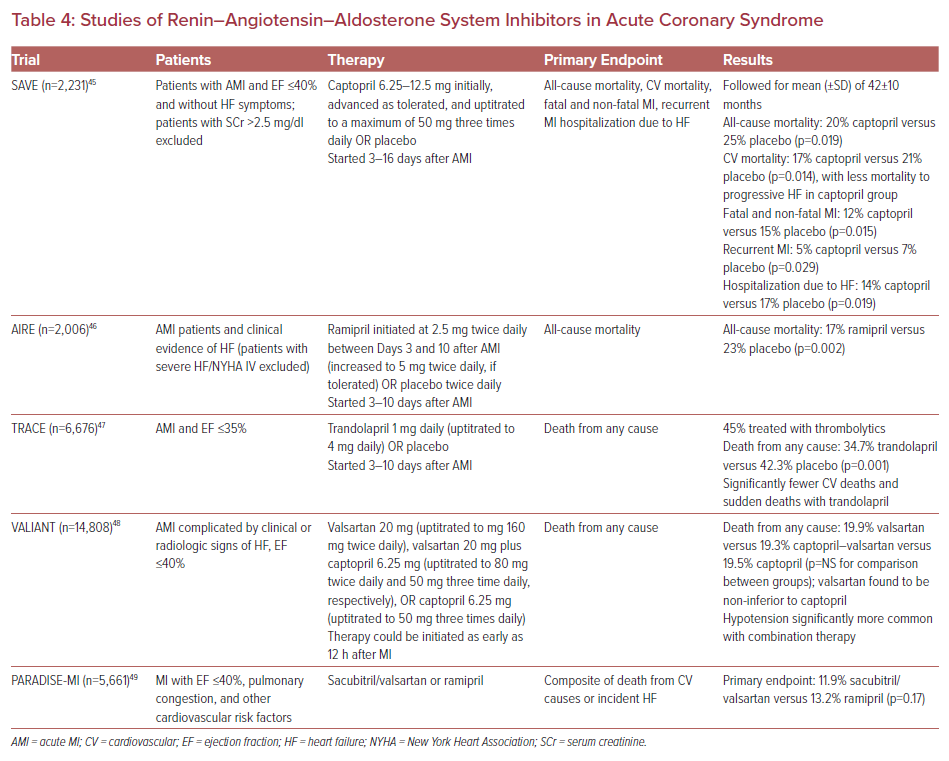
Angiotensin-converting enzyme inhibitors (ACEI) block the conversion of angiotensin I to angiotensin II. Angiotensin II has a number of deleterious effects, such as promoting vasoconstriction and increasing aldosterone levels to increase blood volume, as well as effects on myocardial cell grown and fibrosis. Clinical studies examining the use of ACEI have shown decreases in mortality, heart failure admissions, and progression to heart failure, especially when started early after presentation with ACS and heart failure. Angiotensin II receptor blockers (ARBs) preferentially bind to the angiotensin AT1 receptor, inhibiting the negative effects that may occur following angiotensin II binding to its receptor. Benefits with ACEI and ARBs are seen in patients with or without symptoms of heart failure, and are more pronounced in patients with decreased left ventricular function (Table 4).44–48
The introduction of valsartan/sacubitril and its approval for use in patients with chronic heart failure led to an investigation of this therapy in patients presenting with ACS. The PARADISE-MI was a randomized double-blind controlled trial comparing the effects of valsartan/sacubitril with those of ramipril on cardiovascular death or incident heart failure outcomes.49 Patients with a recent MI with a left ventricular ejection fraction of ≤40%, pulmonary congestion, and a risk-augmenting factor were randomized to valsartan/sacubitril or ramipril. After a median follow-up of 22 months, there was no significant difference between the two groups in cardiovascular death and heart failure outcomes. Adverse events were similar between the two groups, although more patients in the valsartan/sacubitril group experienced hypotension and the ramipril group had a higher incidence of cough.49
The addition of an aldosterone antagonist on top of standard therapy in patients presenting with ACS who have heart failure further decreases the risk of death, as well as heart failure hospitalizations.50 Studies have investigated the initiation of aldosterone antagonists in the early days after MI and, in more contemporary data, within the first few hours after presentation. The addition of the aldosterone antagonist eplerenone was shown to be safe in a low-risk population in one trial, with the main benefits driven by its effects in reducing natriuretic peptide levels, and no difference in major events in a higher-risk population.51,52 Patients who should not receive ACEI, ARBs, or valsartan/sacubitril include those with a previous intolerance to the agents, as well as those with angioedema, hypotension at baseline, serum creatinine concentrations ≥2.5 mg/dl, and those who are pregnant.49 For those placed on an ACEI, ARB, or valsartan/sacubitril plus an aldosterone antagonist, serum concentrations of both creatinine and potassium should be monitored after initiation and periodically to prevent adverse events from renal dysfunction or hyperkalemia. Serious hyperkalemia (serum potassium ≥6.0 mmol/l) occurred at a significantly higher rate in patients assigned an aldosterone antagonist, and was more pronounced in those with decreased renal function.50
β-Blockers
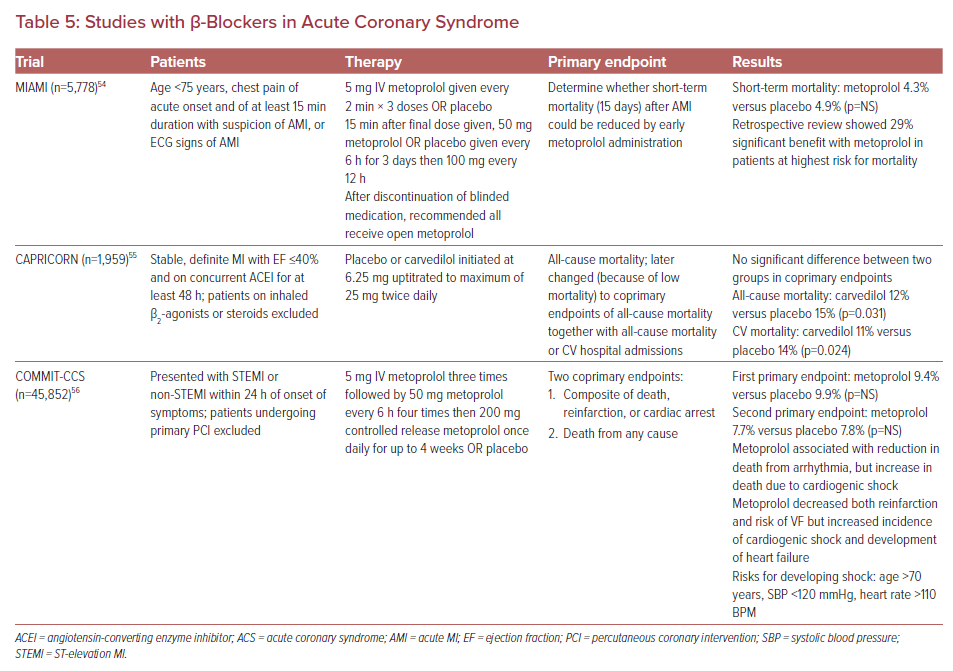
β-Blockers help with ischemia, decreasing the workload of the heart by lowering both heart rate and blood pressure.53 Resting heart rate should be titrated to 50–60 BPM. Early administration is appropriate, provided vital signs are stable and patients do not have symptoms of heart failure, evidence of left ventricular dysfunction, significant bradyarrhythmias, or bronchospastic pulmonary disease. Other benefits of β-blockers include decreased reinfarction and ventricular arrhythmias.53 However, many of the β-blocker trials were performed prior to the use of fibrinolytics and potent antiplatelet therapy. A summary of select β-blocker trials in ACS is provided in Table 5.54–56 These studies found that patients most likely to benefit from the use of β-blockers include those vitally stable with anterior infarcts who present with symptoms of heart failure or develop left ventricular dysfunction, and that β-blockers should be avoided in patients who have signs and symptoms of shock. Outside of these patient populations, the most recent guidelines recommend initiation of oral β-blocker therapy on the day after admission, with uptitration to the maximum tolerated dose based on symptoms and vital signs.57–60
Current Guidelines
Specific guidelines for ACS have not been updated by the American College of Cardiology/American Heart Association (ACC/AHA) for almost 10 years.57,58 Since the publication of those guidelines, cholesterol-lowering guideline updates and updates from the European Society of Cardiology (ESC) have become available.59–61 Most recently, guidelines for coronary artery revascularization have been published by the ACC/AHA, providing some updates on how to manage patients who are revascularized.62 The use of ticagrelor and prasugrel are given a class I recommendation from the ESC, while the ACC/AHA guidelines provide a class II recommendation for ticagrelor and prasugrel use in the setting of ACS.60-62 Current guidelines also give moderate recommendations for the use of cangrelor in the setting of PCI.60-62 The ESC guidelines also recommend an LDL goal of <1.4 mmol/l based on the data presented earlier.60 The ESC guidelines also describe using a lower-intensity strategy with a P2Y12 inhibitor, preferably clopidogrel, along with a direct oral anticoagulant in the setting of AF to minimize the risk of bleeding.59,60
Conclusion
Optimal therapy for ACS includes symptomatic control with nitrates, vasoactive therapies as appropriate, and various antithrombotic strategies depending on how the patient was revascularized. When PCI is performed, aspirin plus a potent thienopyridine, such as ticagrelor or prasugrel, should be chosen to prevent ischemic complications and stent thrombosis. Clopidogrel should be used in select cases, such as patients with a high risk of bleeding or those who require long-term anticoagulation. Cangrelor may be used in select cases where patients have not already received an oral thienopyridine. Anticoagulation should be used with unfractionated heparin because there are no significant advantages in using bivalirudin based on recent studies. Clinicians can consider targeting lower LDL levels, adding ezetimibe or a PCSK9 inhibitor to high-intensity statin therapy. Inhibitors of the renin–angiotensin–aldosterone system should be used in all patients with reduced left ventricular dysfunction, hypertension, and diabetes. β-blockers should be used in patients who have angina or in those with compensated heart failure.