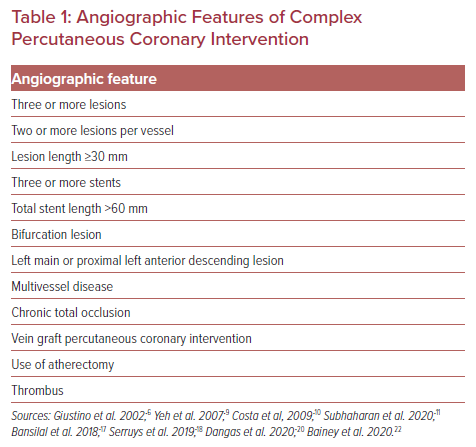
The presence of features commonly considered to characterize a complex percutaneous coronary intervention (PCI) procedure – such as treatment of three or more lesions, implantation of three or more stents, total stent length >60 mm, bifurcation lesion with two stents implanted or treatment of a chronic total occlusion (CTO) – has become increasingly frequent during the last decade.1–3 Additionally, other angiographic features, such as vein graft PCI, multivessel disease or thrombus-containing lesions have also been used to characterize a PCI procedure of increased complexity (Table 1).
Patients undergoing complex PCI are considered to have an elevated risk for ischemic complications since their rate of major adverse cardiovascular events (MACE) has been shown to be significantly higher than that of non-complex PCI patients, especially when multiple complexity features are present.4–6
Dual antiplatelet therapy (DAPT) remains the cornerstone of treatment for patients undergoing PCI with stent implantation; this is intended to reduce atherothrombotic complications, although at the expense of increased bleeding complications. In the setting of complex PCI cases, the use of more potent agents and/or prolonged duration of antiplatelet therapy, although reasonable in terms of ischemic risk containment, raises safety issues, considering the increase of bleeding rates as well as the fact that complex PCI patients may be at an increased bleeding risk per se.7 In this review, we focus on the type and duration of antithrombotic regimens in patients undergoing complex PCI procedures.
Short- Versus Long-term Dual Anti-platelet Therapy
The optimal duration of DAPT in complex PCI patients has yet to be defined, since multiple factors, including patient, clinical and anatomical characteristics, should be taken into consideration before a decision is made.1,8
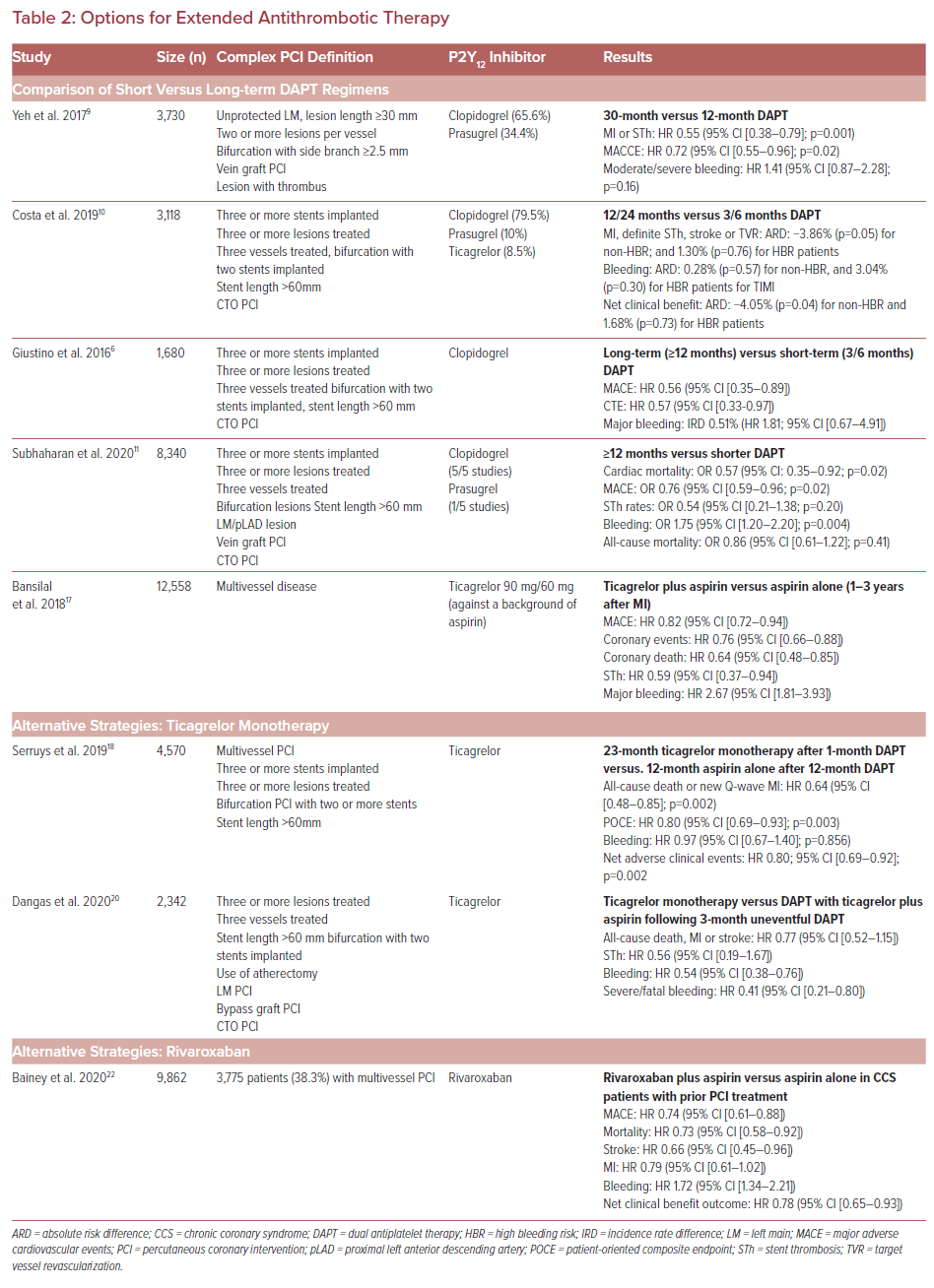
In the DAPT study, 3,730 patients were randomized to receive either prolonged (30-month) or standard (12-month) DAPT following a complex PCI procedure.9 Definition of complex PCI included unprotected left main, lesion length ≥30 mm, more than two lesions per vessel, bifurcation with side branch ≥2.5 mm, bypass vein graft PCI, or a thrombus-containing lesion. Clopidogrel was the P2Y12 inhibitor of choice in 65.6% while prasugrel was used in 34.4% of patients.
Regarding efficacy outcomes, analysis revealed that complex PCI patients in the prolonged DAPT group had a lower risk of major adverse cardiovascular and cerebrovascular events (MACCE) than those in the standard DAPT group (HR 0.72; 95% CI [0.55–0.96]; p=0.02). Additionally, MI and stent thrombosis (STh) rates were also lower in the prolonged DAPT group (HR 0.55; 95% CI [0.38–0.79]; p=0.001). On the other hand, as far as safety outcomes were concerned, prolonged DAPT was not significantly associated with an increase in moderate or severe bleeding events in complex PCI patients (HR 1.41; 95% CI [0.87–2.28]; p=0.16), although this was also evident in the non-complex PCI group (HR 1.78; 95% CI [1.27–2.50]; p<0.001; p for interaction=0.44).9
In the study by Costa et al., high bleeding risk (HBR) status, defined as having a PRECISE-DAPT (PREdicting bleeding Complications in patients undergoing stent Implantation and SubsequEnt Dual AntiPlatelet Therapy) score >25, was evaluated as a potential treatment decision modifier, along with anatomical complexity.10 The study’s population included 3,118 complex PCI patients in total, pooled from eight randomized clinical trials.
Complex PCI was defined as three or more stents implanted, three or more lesions treated, three vessels treated, bifurcation with two stents implanted, total stent length >60 mm, and/or treatment of a CTO. Clopidogrel was used in most patients (79.5%), prasugrel in 10% and ticagrelor in only 8.5% of patients. Compared to short-term (3 or 6 months) DAPT, long-term DAPT (12 or 24 months) was associated with fewer ischemic events in non-HBR patients (absolute risk difference 3.86%; 95% CI [−7.71, 0.06%]; p=0.05) but not in HBR patients (absolute risk difference 1.30%; 95% CI [−6.99, 9.57%]; p=0.76). Regarding safety outcomes, there was a numerical but not statistically significant increase of bleeding rates in non-HBR patients (absolute risk difference 0.28%; 95% CI [−0.46, 1.26%]; p=0.57) as well as in HBR patients (absolute risk difference 3.04%; 95% CI [−2.97, 8.82%]; p=0.30) on long-term DAPT. Interestingly, though, complex PCI was not identified as a factor associated with increased bleeding rates per se.10
Similarly, in a pooled analysis of patient-level data from six randomized controlled trials involving 1,680 complex PCI patients, long-term (≥12 months) DAPT was associated with fewer MACE (adjusted HR 0.56; 95% CI [0.35–0.89]) and coronary thrombotic events (adjusted HR 0.57; 95% CI [0.33–0.97]) than short-term (3 or 6 months) DAPT; all patients were treated with clopidogrel.6 Complex PCI was defined as having three or more stents implanted, three or more lesions treated, three vessels treated, bifurcation with two stents implanted, total stent length >60 mm, or treatment of a CTO.
Interestingly, the beneficial impact of long-term DAPT was progressively greater as the number of complex PCI variables increased, whereas the variable most consistently and strongly associated with elevated ischemic risk was bifurcation PCI with two stents implanted. Of note, all patients were treated with drug-eluting stent (DES) implantation and the effect of long-term DAPT on ischemic outcomes was uniform between early- and new-generation DES. Concerning safety endpoints, via intention-to-treat analysis, there was a trend of higher major bleeding rates observed in the long-term DAPT arm, compared to short-term DAPT (1.03% versus 0.52%; incidence rate difference 0.51%; HR 1.81; 95% CI [0.67–4.91]).6
Finally, the safety and efficacy of long-term (>12 months) DAPT was evaluated in a recent meta-analysis of five studies, including a total of 8,340 complex PCI patients. Complex PCI features included having more than three stents implanted, more than three lesions treated, three vessels treated, bifurcation lesions, total stent length >60 mm, left main or proximal left anterior descending lesion, a vein graft stent, or a CTO. Clopidogrel was the P2Y12 inhibitor of choice in all but one study, where prasugrel was also used.9 Compared to shorter DAPT duration, a DAPT regimen of >12 months was shown to reduce cardiac mortality (OR 0.57; 95% CI [0.35–0.92]; p=0.02) as well as MACE rates (OR 0.76; 95% CI [0.59–0.96]; p=0.02), with no statistically significant difference in STh rates (OR 0.54; 95% CI [0.21–1.38]; p=0.20). However, the anti-ischemic effects of prolonged DAPT came at the cost of an increased rate of bleeding events (OR 1.75; 95% CI [1.20–2.20], p=0.004), although there was no statistically significant difference in all-cause mortality (OR 0.86; 95% CI [0.61–1.22]; p=0.41).11
Apart from extending DAPT with clopidogrel, long-term treatment with ticagrelor (either 60 mg or 90 mg twice daily), on a background of low-dose aspirin, was also tested in PEGASUS-TIMI 54 trial, which included 21,162 patients who had experienced an MI 1–3 years earlier and had additional risk factors. Patients on long-term ticagrelor had a significantly lower risk of the primary efficacy endpoint – a composite of cardiovascular death, MI and stroke – although at a cost of a higher major bleeding risk.12 Ticagrelor effects on both efficacy and safety endpoints were consistent regardless of the presence of prior coronary stenting, although absolute risk reduction tended to be greater in patients without previous PCI because of the already increased baseline ischemic risk.13
Regarding high-risk patient subgroups, the PEGASUS-TIMI 54 subanalyses revealed a greater absolute risk reduction of MACE in patients with diabetes, renal dysfunction and peripheral artery disease (PAD) who were treated with ticagrelor. More specifically, patients with diabetes receiving ticagrelor had an absolute risk reduction of 1.5% compared to 1.1% in patients without diabetes (3-year number needed to treat: 67 versus 91) while, in diabetic patients, cardiovascular death as well as coronary heart disease death rates were reduced by 22% and 34%, respectively, with long-term ticagrelor therapy.14
Patients with renal dysfunction had a greater risk of MACE at 3 years after the index event and consequently experienced a higher absolute risk reduction with long-term treatment with ticagrelor, compared to those without (2.7% versus 0.63%).15 The latter was also observed in the high-risk subset of patients with PAD, who had a greater absolute risk reduction of MACE (4.1%) as well as a lower risk of major adverse limb events (HR 0.65; 95% CI [0.44–0.95; p=0.026]) with long-term ticagrelor.16
Regarding angiographic complexity, patients with multivessel disease had a similar relative risk reduction of MACE with ticagrelor (HR 0.82; 95% CI [0.72–0.94] for pooled ticagrelor versus placebo; p for interaction with patients without multivessel disease = 0.61). Once again, because of the increased baseline risk of MACE in patients with multivessel disease, the absolute risk reduction with ticagrelor tended to be greater than that in patients without (1.43% versus 0.97; 3-year number needed to treat: 70 versus 103).17
In short, prolongation of DAPT beyond 1 year following complex PCI seems a rational strategy towards ischemic risk mitigation in this high-risk subset of patients, although at the cost of a potentially higher risk of bleeding complications.
Alternative Strategies: Ticagrelor Monotherapy
Evaluating the role of potent P2Y12 receptor inhibitors and specifically ticagrelor, a post-hoc analysis of GLOBAL LEADERS trial compared the safety and efficacy of an experimental regimen consisting of a 23-month ticagrelor monotherapy after 1-month DAPT with a reference regimen (12-month aspirin monotherapy after 12-month DAPT – with aspirin and either ticagrelor for acute coronary syndrome (ACS) or clopidogrel for stable coronary artery disease (CAD).18 Complex PCI definition included multivessel PCI, three or more stents implanted, three or more lesions treated, bifurcation PCI with two or more stents, or total stent length >60 mm.
The study’s primary endpoint was the composite of all-cause death or new Q wave MI, whereas the secondary, safety endpoint was BARC (Bleeding Academic Research Consortium) type 3 or 5 bleeding. Out of a total 4,570 complex PCI patients, those following the experimental regimen were found to have a significantly lower risk of the primary endpoint (HR 0.64; 95% CI [0.48–0.85]; p=0.002) as well as the patient-oriented composite endpoint (POCE) – a composite of all-cause death, stroke, MI or revascularization (HR 0.80; 95% CI [0.69–0.93]; p= 0.003) compared to patients under the reference regimen. Interestingly, the risk of bleeding was not statistically different between the two treatment arms (HR 0.97; 95% CI [0.67–1.40]; p=0.856), resulting in a significantly reduced risk of net adverse clinical events in patients under the experimental strategy (HR 0.80; 95% CI [0.69–0.92]; p=0.002).18
Once again, the benefit regarding ischemic events prevention associated with the experimental strategy tended to be greater as the number of complex PCI features increased. However, stratified analyses based on clinical presentation revealed that long-term ticagrelor monotherapy was of benefit mainly to patients with ACS, owing to reductions in both POCE and bleeding rates, resulting in a lower risk of net adverse clinical events (14.07% versus 18.71%; HR 0.73; 95% CI [0.59–0.90]; p=0.003; for interaction with stable CAD patients, p=0.010).18
This finding was also supported by the post-hoc analysis of the GLOBAL LEADERS study, which was dedicated to 3,576 patients undergoing multivessel PCI. Results showed that patients with ACS following the experimental strategy had a lower risk of the primary endpoint (2.95% versus 5.26%; HR 0.55; 95% CI [0.35–0.89]; p=0.014; p for interaction with stable CAD patients = 0.032), driven by a lower risk of all-cause mortality (2.19% versus 4.28%; HR 0.51; 95% CI [0.30–0.87]; p=0.013; p for interaction with stable CAD patients = 0.021), as well as a marginally significant reduced risk of bleeding (HR 0.58; 95% CI [0.33–1.01]; p=0.053; p for interaction with stable CAD patients = 0.334) compared with patients receiving the reference regimen.19
In the same direction, the TWILIGHT-COMPLEX study, a post-hoc analysis of the TWILIGHT trial, evaluated the effects of ticagrelor monotherapy compared to DAPT with aspirin plus ticagrelor in 2,342 event-free patients who had completed 3 months of DAPT. Complex PCI features included three or more lesions treated, three vessels treated, stent length >60 mm, bifurcation with two stents implanted, use of atherectomy device, left main PCI, surgical bypass graft or CTO PCI. Regarding ischemic endpoints, the two treatment regimens had similar results, in terms of both the composite of death, MI or stroke (3.8% versus 4.9%; absolute risk difference 1.1%; HR 0.77; 95% CI [0.52–1.15]) and STh (0.4% versus 0.8%; absolute risk difference −0.4%; HR 0.56; 95% CI [0.19–1.67]) rates. Nevertheless, patients under the ticagrelor monotherapy regimen were found to have a lower risk of a BARC type 2, 3, or 5 bleeding during the first year after the index PCI (4.2% versus 7.7%; absolute risk difference −3.5%; HR 0.54; 95% CI [0.38–0.76]), and severe or fatal bleeding rates were also significantly lower than in patients in the DAPT arm (1.1% versus 2.6%; absolute risk difference −1.5%; HR 0.41; 95% CI [0.21–0.80]). Of note, the effect of ticagrelor monotherapy versus DAPT for the endpoint of death, MI, or stroke was consistent across all variables of complex PCI as well as after stratification based on the presence of progressive number of complex PCI features.20
To sum up, recent evidence points to the superiority of long-term ticagrelor monotherapy following a short course of DAPT concerning safety outcomes with no evidence of reduced efficacy, providing an alternative strategy towards bleeding risk mitigation in the high-risk population of complex PCI patients.
Alternative Strategies: Rivaroxaban
Another strategy of antithrombotic treatment extension involves the addition of an anticoagulant to aspirin for long-term prevention of atherothrombotic events. The COMPASS trial investigated the safety and efficacy of rivaroxaban 2.5 mg twice daily on top of aspirin in patients with stable CAD or PAD. Patients receiving rivaroxaban plus aspirin had a lower rate of MACE (HR 0.76; 95% CI [0.66–0.86]; p<0.001) than those on aspirin alone, although at the expense of an increase in major bleeding events (HR 1.70; 95% CI [1.40–2.05]; p<0.001).21
In a prespecified subgroup analysis of COMPASS, similar to non-PCI patients, stable CAD patients treated with PCI had a reduced rate of MACE when treated with rivaroxaban plus aspirin versus aspirin alone (4.0% versus 5.5%; HR 0.74; 95% CI [0.61–0.88]) as well as lower rates of mortality (2.5% versus 3.5%; HR 0.73; 95% CI [[0.58–0.92]) and stroke (0.9% versus 1.4%; HR 0.66; 95% CI [0.45–0.96]).22 There was also a modest trend toward lower MI rates in PCI patients under rivaroxaban plus aspirin in comparison with aspirin alone, although statistical significance was not reached (2.2% versus 2.7%; HR 0.79; 95% CI [0.61–1.02]). Of interest, the results were consistent in patients with previous single-vessel and multivessel PCI (for interaction, p=0.73).
Regarding safety, the combination of rivaroxaban plus aspirin was associated with a higher rate of major bleeding events compared to aspirin alone (3.3% versus 2.0%; HR 1.72; 95% CI [1.34–2.21]), with no difference in intracranial bleeding, fatal bleeding or bleeding into a critical organ. Therefore, the risk of the composite net clinical benefit outcome (cardiovascular death, stroke, MI, fatal bleeding, or symptomatic bleeding into a critical organ) was lower in the rivaroxaban plus aspirin versus aspirin-alone arm (4.6% versus 5.9%; HR 0.78; 95% CI [0.65–0.93]). Of note, 62.8% of patients had undergone a PCI performed three or more years ago and 32.3% had undergone PCI 1–3 years before randomization. Interestingly, though, the benefit of rivaroxaban plus aspirin on MACE reduction among PCI-treated patients was consistent irrespective of the time PCI was performed (1–10 years before randomization) or the presence or absence of previous MI.22
Detailed presentation of studies investigating antithrombotic regimens of different type and duration are presented in Table 2.
Guideline Recommendations and Future Considerations
Although not specifically defining complex PCI, American College of Cardiology/American Heart Association guidelines consider some procedural and anatomical features, such as greater stent length or bifurcation lesions, as high ischemic risk factors that could potentially advocate for a prolonged DAPT regimen; this would be for an additional 18–36 months after an initial 6–12 months of DAPT.8 On the other hand, European Society of Cardiology (ESC) guidelines propose that prolonged DAPT (>6 months) may be considered (class IIb recommendation) for patients undergoing complex PCI, defined as treatment of three or more lesions, three or more stents implanted, stent length >60 mm, bifurcation PCI with two stents implanted or treatment of a CTO.1 However, guidelines on chronic coronary syndromes suggest extension of antithrombotic therapy in patients with high ischemic risk (defined as diffuse multivessel CAD plus at least one of the following: diabetes requiring medication; recurrent MI, PAD, or chronic kidney dysfunction) and without high bleeding risk (class IIa recommendation).23
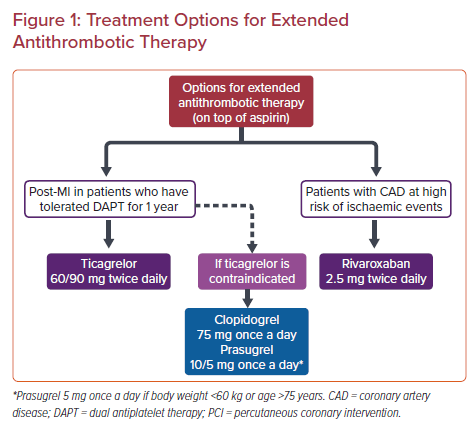
Recently published European guidelines regarding the management of ACS in patients presenting without persistent ST-segment elevation also recommend extended treatment (>12 months) with a second antithrombotic agent on top of aspirin, either rivaroxaban 2.5 mg or ticagrelor (or clopidogrel or prasugrel in cases of contraindication to ticagrelor) in patients at a high risk of ischemic events and without an increased risk of bleeding (class IIa recommendation). High-risk cases include patients with complex CAD undergoing PCI with implantation of three or more stents, treatment of three or more lesions, stent length >60 mm, history of left main or bifurcation stenting with two stents, treatment of a CTO or stenting of the last patent vessel, as well as patients with multivessel CAD or history of stent thrombosis under antiplatelet therapy, among others.24 Treatment options for extended antithrombotic therapy are presented in Figure 1.23,24
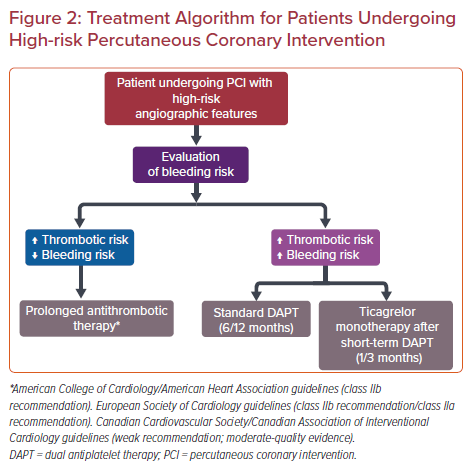
Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology guidelines also consider the prolongation of DAPT beyond 6 months in stable CAD patients with high-risk clinical or angiographic features who are not at a high risk of bleeding (weak recommendation; moderate-quality evidence). High-risk angiographic features include three or more stents implanted, three or more lesions stented, use of a biodegradable vascular scaffold, total stent length >60 mm, bifurcation treated with two stents, CTO PCI, left main or proximal left anterior descending artery stenting as well as multivessel PCI.25 A concise algorithm regarding potential treatment strategies in patients undergoing high-risk PCI procedures is presented in Figure 2.
The use of potent P2Y12 receptor inhibitor in patients undergoing complex PCI, although a potentially promising strategy towards ischemic risk mitigation, has not been evaluated thoroughly since in most published studies clopidogrel was the P2Y12 receptor inhibitor of choice. Nevertheless, the 2018 ESC/European Association for Cardio-Thoracic Surgery guidelines on myocardial revascularization state that prasugrel or ticagrelor may be used in high-risk elective PCI, such as left main stenting and CTO procedures (class IIb recommendation).26
Additionally, the 2019 ESC guidelines on chronic coronary syndromes suggest the use of prasugrel or ticagrelor, at least as initial therapy, in specific high-risk situations of elective stenting – such as suboptimal stent deployment or other procedural characteristics associated with high risk of STh, complex left main stem, or multivessel stenting – or if DAPT cannot be used because of aspirin intolerance.23
However, an analysis of the PROMETHEUS study comparing prasugrel with clopidogrel in 9,735 patients undergoing complex PCI for ACS showed that prasugrel administration was inversely proportional to procedural complexity, revealing the hesitancy of physicians to prescribe potent P2Y12 inhibitors in high-risk situations. Nevertheless, the use of prasugrel was associated with a significantly lower risk of MACE at 1 year (HR 0.79; 95% CI [0.68–0.92]; p=0.002), compared to clopidogrel.27
In the same spirit, the ongoing SMART-ATTEMPT trial (NCT04014803) is evaluating the use of potent P2Y12 inhibitors in elective complex PCI patients.
Conclusion
The increasing prevalence along with the elevated atherothrombotic risk of complex PCI cases in everyday clinical practice dictate the need to identify the optimal antithrombotic treatment strategy for this high-risk patient population, through an individualized approach that would result in a balanced thrombotic/bleeding risk prevention.
Although prolonged DAPT duration seems to reduce ischemic complications, the associated potentially elevated bleeding risk hinders a universal adoption of extended DAPT regimens in complex PCI patients. On the other hand, a regimen consisting of long-term ticagrelor monotherapy after a short course of DAPT seems promising, especially in patients with a high bleeding risk.
Therefore, the role of potent P2Y12 receptor inhibitors in the management of complex PCI patients needs to be further evaluated as part of antiplatelet treatment regimens of various combinations and durations.
Finally, the addition of low-dose anticoagulant on top of aspirin has shown promising results in the long-term prevention of ischemic events in selected stable CAD patients with a history of PCI, even several years after the index procedure.