Transcatheter aortic valve replacement (TAVR) is an established therapeutic option for patients with symptomatic, severe aortic stenosis (AS) across the spectrum of surgical risk. With an increasing desire to expand TAVR to younger patients with low surgical risk, there have been continued technological advancements to optimize TAVR results to minimize post-procedural complications and address challenges when considering using TAVR in a younger population with a longer life expectancy. This paper will review the role of multimodality imaging to determine precise sizing and positioning of transcatheter heart valves (THVs) to avoid post-procedural complications such as paravalvular leak, prosthesis–patient mismatch, and conduction abnormalities. We will discuss innovative fluoroscopic cusp-overlap and shallow deployment techniques to minimize interaction of THVs with the conduction system and highlight recent data on the significance of commissural alignment to facilitate coronary reaccess and valve-in-valve (ViV) procedures following TAVR.
Modalities of Imaging for Pre-procedural Size Assessment
Meticulous pre-procedure planning is an important step to clearly understand each patient’s unique anatomy and to facilitate optimal procedural workflow. A multimodality imaging approach, combining transthoracic echocardiography and multi-slice CT (MSCT), with occasional use of transesophageal echocardiography in select situations, is a better approach to predict pre- and post-procedural complications.1,2
MSCT identifies precise anatomical details including distribution and localization of calcification and allows for a more accurate delineation of the access route (transfemoral versus other). It also allows for detailed visualization of the left ventricular outflow tract (LVOT) and coronary ostia. MSCT is the standard imaging modality for aortic annulus measurement to determine valve size and positioning. One potential limitation of MSCT is the risk of contrast-induced kidney injury, although low-contrast protocols have been used to minimize this risk, and with accurate pre-procedure planning, TAVR can be performed using limited or no contrast dye.3 Although 3D transesophageal echocardiography (3D-TEE) had previously been used for valve size assessment, the estimation of annulus size and area by 3D-TEE is reported to be significantly smaller when compared to MSCT and can be considered only when MSCT is contraindicated.3,4 Nevertheless, it remains an important adjunct to peri-procedural imaging in select cases.
Paravalvular Leak: Role of Multi-slice CT and Accurate Device Selection
Historically there has been a higher incidence of paravalvular leak (PVL) with TAVR compared with surgical aortic valve replacement (SAVR), and this has been associated with worse outcomes.5,6 Mismatch of the valve annulus and prosthesis diameter sizes, device landing zone calcification, and suboptimal device implantation have been identified as the major predictors of PVL.7.8 With implementation of pre-procedural MSCT instead of TEE and more accurate sizing of THVs, the incidence of PVL has been significantly reduced.9 Current algorithms continue to use oversizing to ensure complete coverage of the aortic annulus. A −5% to +20% oversizing may be used with the balloon-expandable valve (BEV) and a perimeter oversizing of 7–30% for the self-expanding valve (SEV).10
While the incidence of PVL was observed to be higher in early generation valves, the rate has improved with the availability of a wider range of valve sizes and with the development of newer generation valves that have been engineered to provide better sealing mechanisms.11
The PARTNER 3 trial reported similar rates of moderate to severe PVL with the BEVs compared to SAVR (0.6% versus 0.5%).12 However, the incidence of moderate to severe PVL was reported to be higher with SEVs as seen in the Medtronic low-risk study (3.5% versus 0.55%).13 Medtronic CoreValve Evolut PRO is the latest generation of self-expanding valves (SEVs) that has been redesigned with a pericardial wrap on the distal outer stent to improve annular sealing and has been shown to result in none or trace/mild PVL in >95% of patients at discharge and at 30 days.14 With continued use of current generation THV platforms with improved sealing mechanisms and increasing operator experience, the incidence of anything more than mild PVL is relatively low.
Prosthesis–Patient Mismatch
Hemodynamics of most prosthetic valves are often sub-optimal to those of the normal native valve, and a significant proportion of patients undergoing aortic valve replacement have high residual transprosthetic pressure gradients due to prosthesis–patient mismatch. Prosthesis–patient mismatch occurs when the effective orifice area (EOA) of the prosthesis is too small relative to the patient’s body surface area. It is considered absent or not clinically significant when the indexed EOA is >0.85 cm2/m2, moderate when it is between 0.65 and 0.85 cm2/m2, and severe when it is <0.65 cm2/m2.15
Although there is conflicting evidence regarding the impact of prosthesis–patient mismatch on clinical outcomes, likely because of methodological differences across studies and the patient population being studied, a large meta-analysis of 34 observational studies comprising 27,186 patients demonstrated an increased mortality in patients with prosthesis–patient mismatch following SAVR.16 Some studies of SAVR have suggested that the impact of prosthesis–patient mismatch on mortality was only observed in younger patients (aged <60–70 years) with a more active lifestyle.17,18
More recently, in an analysis of 62,125 patients from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (TVT) Registry who received TAVR, Herrmann et al. found that severe prosthesis–patient mismatch was associated with increased mortality and heart failure rehospitalizations at 1 year after TAVR.19 The analysis also identified important predictors of prosthesis–patient mismatch with the highest odds ratios in those receiving smaller prostheses (≤23 mm diameter), larger patients and those undergoing a ViV procedure. Although the incidence of prosthesis–patient mismatch in TAVR is generally lower than in SAVR, the study by Herrmann et al. emphasizes the importance of preventing prosthesis–patient mismatch at the time of TAVR, considering the expansion of TAVR to younger and low surgical risk patients.20
Tang et al. evaluated the incidence, predictors, and outcomes of prosthesis–patient mismatch in patients implanted with supra-annular valves in both de novo TAVR and transcatheter aortic valve (TAV) in-surgical aortic valve (SAV) in the TVT Registry.21 Severe prosthesis–patient mismatch was found in 5.3% of patients undergoing de novo TAVR and 27% of patients undergoing TAV-in-SAV. The study demonstrated that an aortic annular diameter of <20 mm in de novo TAVR and TAV-in-SAV patients was a significant predictor of severe prosthesis–patient mismatch (p<0.001 for both). Although the 23 mm valve was also associated with severe prosthesis–patient mismatch, it was generally reserved for patients with annular diameters <20 mm.
Considering increasing awareness regarding implications of prosthesis–patient mismatch, it becomes important to identify patients at increased risk and adopt techniques to minimize risk. SEVs have shown a consistent reduction in prosthesis–patient mismatch incidence in both large and small annuli compared with SAVR.22 SEVs are supra-annular in position allowing for a larger EOA which can prevent prosthesis–patient mismatch when compared with BEVs, which have an intra-annular position.
In a propensity score-matched analysis comparing SEV and BEV, Okuno et al. found that the rate of prosthesis–patient mismatch was significantly lower in SEV compared with BEV (33.5% versus 46.9%; p=0.004; severe prosthesis–patient mismatch, 6.7% versus 15.6%; p=0.003).23 The effect was consistent across annulus sizes and the difference was driven by larger patients with body surface area >1.83 m2.
Jilaihawi et al. demonstrated that optimal positioning with a reduced LV depth of a self-expanding prosthesis was associated with a reduction in moderate and severe prosthesis–patient mismatch from 48% to 16%.24 Data from the TAVI-SMALL registry suggest that post-dilatation and perimeter ratio >15% protects against prosthesis–patient mismatch when patients with small annuli are treated.25 SAVR with aortic root enlargement to accommodate a larger surgical bioprosthesis may be preferred to TAVR with a 23 mm supra-annular TAV.21 Similarly, patients with pre-existing severe prosthesis–patient mismatch post-SAVR who are being considered for TAV-in-SAV will likely continue to have severe prosthesis–patient mismatch following TAVR.21 Bioprosthetic valve fracture using a high-pressure balloon has also been shown to result in reduced residual transvalvular gradients and increased valve EOA.26
Conduction Abnormalities and the Significance of Implantation Depth
Conduction system abnormalities are the most common complication following TAVR and are associated with increased morbidity and mortality, length of hospital stay, and cost of care. When devising strategies to minimize injury to the conduction system, it is important to have a basic understanding of the conduction system in relation to aortic valve anatomy. The aortic annulus is an oval shaped, crown-like leaflet structure which, when seen in 3D, takes the form of a three-pronged coronet with three anchor points from the supporting ventricular structure.1,2 During annular sizing, the aortic annulus is measured at the level of the lowest hinge point of aortic leaflets at the virtual/inferior basal ring during systole.2
The atrioventricular (AV) node is located at the triangle of Koch in the right atrium. The AV node extends as the AV bundle/the His bundle, which runs on the left side of the central fibrous body prior to entering the ventricular septum. It is then divided into left and right bundle branches. The left bundle branch (LBB) and His bundle pass closely between the non-coronary cusp (NCC) and the right coronary cusp (RCC) at the base of the aortic commissure. Due to the concurrent anatomical relationship between the aortic annulus and LBB, surgical or mechanical handling of the aortic root may cause conduction abnormalities secondary to direct injury, inflammation, edema, or ischemia.27 Strategies to minimize conduction system injury focus on minimizing interaction of the valve and delivery system with the membranous and muscular septum where the conduction fibers are most superficial.
Both the balloon-expandable SAPIEN (Edwards) and the self-expanding CoreValve (Medtronic) have evolved to reduce the risk of peri-procedural complications including conduction abnormalities. Historically, data reveals higher incidence of permanent pacemaker implantation (PPMI) in early generation Medtronic self-expanding CoreValve compared to the balloon-expandable SAPIEN valve. A considerable difference in PPMI rate has been noticed in the current generations of both SAPIEN and CoreValve. The rate ranged from 2.3% and 28.2% in early generation SAPIEN valves while in the newer generation SAPIEN 3 the PPMI rate has been between 4.0% and 24.0%.28 The PPMI rate with early generation CoreValve was significantly higher at 16.3–37.7%. With enhanced features of next generation Evolut R, allowing repositioning and recapturing during valve deployment, the PPMI rate has gone down to 14.7–26.7%, albeit remaining higher than the SAPIEN 3 THV.28 Despite the iterative technological advancements in THV platforms, the rate of conduction abnormalities and the need for PPMI has remained higher in TAVR compared to SAVR; the Medtronic low risk study reported the PPMI rate as 17.4% in TAVR patients compared to 6.1% among SAVR patients while in the Partner 3 trial, the PPMI rate was 6.6% in the TAVR group compared to 6.1% in the SAVR group.12,13 PPMs have been associated with significantly longer post-procedure hospitalization and higher rates of repeat hospitalizations.29 More recent data also suggests higher all-cause mortality and reduced left ventricular ejection fraction in patients who received PPM post-TAVR.30
Among other risk factors, such as pre-existing right bundle branch block and asymmetric calcification patterns, low implantation depth of the THV has been identified as an important predictor of conduction abnormalities leading to PPMI following both BEVs and SEVs.31 In an analysis of 229 patients who underwent TAVR with SAPIEN 3, Mauri et al. demonstrated that implantation depth was significantly greater in patients requiring PPMI than in those without the need for PPMI (ventricular part of the stent frame, 29 ± 11% versus 21 ± 5%; p<0.001) and described that the ventricular part of the stent frame of 25.5% as the best threshold to discriminate between patients with high and low risk for PPMI.32 In a multicenter trial of 60 patients who underwent TAVR with Evolut R, Manoharan et al. found that 11.7% of the patients required PPM.33 When the depth of implant was reviewed, the average depth for those who received a PPM (left coronary cusp [LCC] 9.4 ± 3.1 mm; non-coronary cusp [NCC] 8.1 ± 3.5 mm) was significantly deeper than that of patients who did not receive a new pacemaker (LCS 4.3 ± 2.5 mm; NCS 3.3 ± 2.5 mm; p<0.0001 for both comparisons).
Jilaihawi et al. described the variation in PPM rates depending on the length of the membranous septum and the depth of the THV.34 Their series suggested that when a THV is deployed below the membranous septum, the risk of PPMI increased. The OR of PPMI increased significantly with the difference between membranous septal length and implantation depth (p<0.001).
ViV TAVR has become a popular alternative to reoperative surgery in degenerative surgical aortic bioprostheses. In a contemporary large series of ViV TAVR patients, the rate of post-procedural PPMI was found to be relatively low (6.4%) compared to a PPMI rate of nearly 15% associated with TAVR in native aortic valves. A significant decrease in the incidence of PPMI with the use of new-generation THVs (4.7% versus 7.4%; p=0.017), mainly related to a reduced PPMI rate with the Evolut R/Pro versus CoreValve (3.7% versus 9.0%; p=0.002) was noted.35 The mechanical protection of the degenerated surgical bioprosthesis structure against a potential compression of the conduction system by the THV has been suggested as the main factor preventing conduction abnormalities after ViV TAVR.35 Also, recent studies have shown a lower risk of elevated gradients and improved valve hemodynamics with high implantation of THV in ViV TAVR.36,37
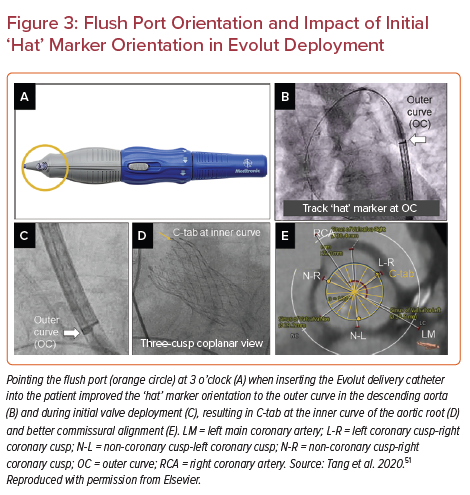
The instructions for CoreValve THV recommend an implantation depth of 3–5 mm below the annular plane. However, the assessment of true implantation depth remains challenging. In our recent analysis of 130 patients from the Medtronic low risk study who underwent both aortography and MSCT post-TAVR, we found a limited correlation (r=0.69) between implant depths by aortography versus MSCT, with aortography underestimating the depth of the implant. The risk of PPMI was significantly higher with deeper valve implantation assessed by MSCT (p=0.04), but not by aortography (p=0.12).38 The study highlights the need to develop techniques for precise measurement of implantation depth. The double S-curve and the cusp-overlap are innovative techniques that have allowed us to achieve a more accurate measurement of implantation depth, thereby allowing shallower valve deployment with high rates of success and minimal procedural complications.
Double S-curve
An important step in the deployment of SEVs is identifying the optimal fluoroscopic projection where the aortic annulus plane is orthogonal to the delivery catheter to remove parallax. MSCT images can be used to define the direction of structures and produce an optimal projection curve. The intersection point of the S-curve of the aortic annulus and that of the delivery catheter on the double S-curve gives the angiographic projection where the aortic annulus and the delivery catheter appear perpendicular. This is defined as the optimal projection for implantation as it eliminates foreshortening of the delivery catheter and the aortic annulus. Therefore, in this view, the position of the THV relative to the delivery catheter in NCC can be precisely measured as no foreshortening occurs. The optimal projection curve of the aortic valve annulus can be determined by pre-procedural MSCT; however, the optimal projection curve of the delivery catheter needs to be generated during the procedure.39,40
Cusp-overlap Technique
The cusp-overlap technique on fluoroscopy is a novel method that our center has used to achieve an optimal projection for deployment. This technique involves positioning the C-arm to superimpose the LCC over the RCC, and isolate the NCC, which is the most inferiorly oriented cusp in the LVOT. The fluoroscopic angulation for cusp-overlap is determined on pre-procedural MSCT. As compared with the double S-curve, the cusp-overlap technique has the advantage of not requiring any additional intra-procedural imaging. The right coronary/non-coronary commissure is displayed in the center of the fluoroscopic image and allows the operator to deploy the valve in an attempt to avoid the muscular septum and the superficial conduction fibers. Below the right/non-coronary commissure, the membranous septum houses the conduction system. If the THV is deployed below the membranous septum, there is a greater chance of interaction with the conduction system.
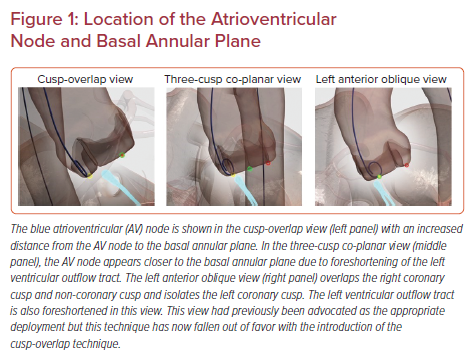
In a non-cusp-overlap view, the LVOT is foreshortened, leading to the false perception that THV is in a shallower position relative to insertion of the NCC. The cusp overlap view minimizes foreshortening and elongates the LVOT and highlights the NCC/RCC commissure in the center of the fluoroscopic view. Given this projection of the LVOT, there is also a greater distance between NCC insertion and the compact AV node (Figure 1). This fluoroscopic view leads to a more precise 3 mm implantation depth and minimizes the risk of interaction with the conduction system.
In a recent single-center, non-randomized retrospective study, Ben-Shoshan et al. assessed the concordance between transcatheter aortic valve angles generated by the double S-curve and cusp-overlap techniques.41 The study included 100 consecutive patients undergoing TAVR with a self-expanding device planned by MSCT. TAVR was performed using the double S-curve model. Optimal projection according to the cusp-overlap technique was retrospectively generated by overlapping the right and left cups on the MSCT annular plane. The study demonstrated no significant differences in average coordinates between the double S-curve and cusp-overlap methods. The angular coordinates were noted to be in the same imaging quadrant 92% of the time with the vast majority (>80%) being in the right anterior oblique (RAO)/caudal (CAU) quadrant (Figure 2). The RAO and CAU projection of the left heart mostly represents a three-chamber view, which provides maximal elongation of the aortic root and LVOT and of the delivery catheter. Avoidance of foreshortening of the LVOT and delivery catheter provides a realistic perception of THV implant depth. The study demonstrates that the cusp-overlap method can be used as a reliable surrogate for the double S-curve model to define optimal projections for TAVR, obviating the need for intra-procedural image processing.
We have incorporated several procedural steps in addition to the cusp-overlap technique to further minimize the interaction with the conduction system during TAVR:
- We emphasize a top-down approach for deployment of the THV, starting with the catheter marker band positioned at the mid portion of the pigtail in the NCC.
- Once the capsule is retracted, the inflow of a nitinol prosthesis advances across the annulus and is positioned at 3 mm below. This maneuver avoids traumatic advancement of the bioprosthesis into the ventricle with inflow flaring deeper within the left ventricle, which results in subsequent maneuvers to retract the catheter, thus moving the transcatheter valve shallower to a more aortic position, and increasing interaction of the nose cone of the delivery system with the membranous septum.
- We use a stiffer, double-curved, Lunderquist wire (Cook Medical) in most cases to hold the wire in position in the non-coronary right commissure and begin the prosthesis deployment along the posterior aspect of the annular plane. Although any wire can be used to support the delivery system, the stiffer wire may achieve more symmetrical deployment and is especially valuable when deploying larger sized THVs. Additional care should be taken when using this wire because of its stiffness to reduce the risk of ventricular injury. The use of this wire is being tested prospectively in the OPTIMIZE PRO post-market analysis (NCT04091048).
- Using the cusp-overlap view to maintain reference to the native annular plane, the marker band on the THV delivery catheter tends to lose parallax when approaching the valve plane. The loss of parallax of the marker band is the result of the delivery catheter following the stiff left ventricular wire that is generally positioned in the NCC/RCC commissure and confirms alignment of the prosthesis with the annular plane. This approach may lead to more confidence in the initial positioning of the THV in relation to the insertion of the NCC and a better assessment at the point of no recapture.
- We favor sufficient pacing (140–180 BPM) during the deployment to minimize cardiac output and occurrence of premature ventricular contraction burden, allowing for stable deployment of the bioprosthesis.
- Finally, once we are at 80% deployment, we rotate the gantry to a LAO projection to visualize the LCC and ensure that the inflow is not supra-annular. We aim for an implantation depth of 3 mm and no deeper than 5 mm below the NCC to reduce the risk of conduction disturbance. We occasionally aim for a shallower deployment in patients at high risk for conduction system abnormality but recommend recapture for bioprothesis positions less than 1 mm or more than 5 mm within the ventricle.
- Once final positioning is confirmed, we retract the left ventricular wire, centralize the nose cone, and slowly release the delivery catheter from the bioprothesis by releasing the frame paddles. We are careful to avoid interaction of the delivery catheter and the bioprosthesis as the delivery catheter is retracted into the aorta.
Our institution adopted the cusp-overlap technique in October 2015 and since then witnessed a significant decline in the post-TAVR PPMI rates with the Medtronic CoreValve and Evolut platforms. In the Medtronic low risk trial, the rate of PPMI 30-days post-TAVR was 17.4%, with wide site-specific variability.13 Our single-center experience of using the cusp-overlap technique found that only 1 in 65 patients (1.5%) required a PPM. As the highest enrolling center in the study, we attribute this difference to the comprehensive use of the cusp-overlap technique at our institution.
The use of 34 mm Evolut R THV has been identified as a risk factor for PPMI with the associated rate of 16.7% in the TVT registry. From June 2016 to July 2019, we implanted the 34 mm Evolut R THV in 134 consecutive patients without a previous pacemaker, using the fluoroscopic cusp-overlap technique. It was successfully performed in 88% of the patients, with the remainder having near overlap or not having it done due to steep gantry angles. The in-hospital rate of new-onset LBB block was 10.9% and the 30-day PPMI rate was noted to be 5.2%, lower than the previously published rates.42 The study demonstrates that the risk of conduction abnormalities and PPMI with the 34 mm Evolut R can be significantly reduced using the cusp-overlap technique.
We also began formal training of the technique for physicians in Latin America in July 2018 and evaluated the clinical outcomes of Evolut THV implantation until October 2019. Fourteen implanting physicians from seven countries performed consecutive procedures on 114 patients. Of these, 105 patients did not have a prior PPM. The in-hospital rate of new PPMI was 5.7%. At 30-day follow-up that included 85 patients, no additional patients required a new PPM.43 The study demonstrates consistent results of lower rates of PPMI after Evolut THV implantation with the adoption of cusp-overlap technique across different centers.
Our experience with using cusp-overlap technique to implant Medtronic CoreValve and Evolut platforms has revealed a consistently reduced risk of conduction abnormalities and lower incidence of PPMI after TAVR compared to prior studies. The lower rates of PPMI using the cusp-overlap technique have also been reproducible by physicians from seven different Latin American countries. The OPTMIZE PRO study (NCT04091048) will expand on this with further data regarding the safety and efficacy of the technique across multiple centers.
High Implantation of SAPIEN 3
As discussed before, a low implantation depth is associated with an increased incidence of conduction abnormalities with BEVs. Traditionally, BEVs are deployed using a three cusp co-planar view in an LAO projection. In a recent paper, Sammour et al. demonstrated a novel technique to deploy SAPIEN 3 in RAO/CAU projection instead of the conventional co-planar view with LAO projection to achieve a higher implantation depth, thereby minimizing the risk of conduction abnormalities.44
The authors described three procedural modifications:
- Deploying the valve in the RAO/CAU view to remove the parallax.
- Positioning the valve by aligning the radiolucent line that is located at the superior aspect of the lowest set of stent struts of the crimped valve at the base of the NCC.
- Using a straight flush catheter instead of a pigtail to mark the native annulus, reducing the risk of trapping the pigtail catheter between the sinus and the valve.
With the use of this novel high deployment technique (HDT), the authors demonstrated significantly smaller implantation depths (1.5 ± 1.6 mm versus 3.2 ± 1.9 mm; p<0.001) and lower rates of PPMI (5.5% versus 13.1%; p<0.001) compared to the conventional technique without a statistically significant increase in adverse clinical outcomes, aortic regurgitation, or valve embolization between the two groups.
This study signifies the understanding of optimal angiographic projections necessary to achieve a reduction in parallax and valve deployment aligned with the radiolucent line in the RAO/CAU projection to allow greater precision and consistency in a shallower deployment.
Limitations of Shallow Implantation Depth
While shallow implantation depth is associated with lower rates of PPMI, there are potential drawbacks of shallow deployment of THV, with a risk of valve embolization being the most serious. With the HDT described above, one of 406 patients (0.2%) had valve embolization. Although the risk remains low (<1%), operators who use this method must be adept with techniques to manage valve embolization, either by migrating the THV to a safe location in the aorta using a balloon (with SAPIEN) or via snare (with CoreValve) while working quickly to deploy a second prosthesis.
Shallow implantation may risk or exclude future ViV options for certain low-risk patients, as a ViV approach would push up the leaflets of the first THV to create a neoskirt that can potentially occlude native coronaries.
Coronary reaccess is another major concern, given that about two-thirds of TAVR patients have pre-existing coronary artery disease. In patients with lower surgical risk, it is essential to consider future coronary access. In the RE-ACCESS study of 300 enrolled patients, Barbanti et al. showed that a total of 23 patients (7.7%) had an impediment to coronary ostia post-TAVR (4.7% in the left coronary artery, 4.0% in the right coronary artery), compared with no cases of unsuccessful cannulation before TAVR.45 Unsuccessful cannulation occurred in 22 of 23 cases with the use of Evolut R/PRO THVs (17.9% versus 0.4%; p<0.01). The use of Evolut R/PRO THVs, sinus of Valsalva oversizing and high implantation depth (with a cut-off value <6 mm) were found to be independent predictors of unsuccessful coronary cannulation after TAVR.
When an Evolut THV is implanted high, the narrow portion of the frame could potentially end up above the origin of the coronary arteries, resulting in ostia facing the inflow part of the frame, which is covered by the pericardial skirt. Hence, Evolut THV bioprosthesis needs careful selection for each patient to balance the risk for unsuccessful coronary cannulation and the risk for conduction disturbances.
Commissural alignment avoids covering the coronary ostia with the THV struts. However, a shallow deployment – particularly in patients with narrow sinus or low coronaries – may still complicate future coronary access.46
Commissural Alignment
In SAVR, native calcified aortic valve leaflets are excised and the bioprosthetic valve is implanted in an anatomic orientation where the commissures of the bioprosthetic valve are aligned with the commissures of the native valve. In TAVR, the native valves remain in situ and the THV neo-commissures are oriented randomly. The commissural misalignment leads to a varying degree of overlap between the neo-commissural posts and the coronary ostia.47
The coronary arteries are easily accessible when coronary ostia are situated distal to the THV stent frame. This may be challenging with supra-annular THVs with tall stent frames because of the need to cross the stent frame to access the coronary ostia but may be less of a problem when using THVs with short stent frame heights. Coronary reaccess can also be hindered by the random location of THV commissural posts relative to the coronary ostia as well as the native leaflets.48 Percutaneous coronary intervention after TAVR has been reported in 1.9–5.7% cases.49,50 As TAVR continues to expand to younger people and those with a lower surgical risk, achieving commissural alignment in THV becomes imperative to facilitate future coronary access.
Commissural alignment is also significant when ViV is considered. In patients with an increased risk of coronary artery obstruction with ViV, bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) can be performed to reduce coronary flow. However, if the initial THV commissure is not aligned and apposes the lacerated leaflet, the BASILICA technique may fail to prevent coronary obstruction.51
In the ALIGN-TAVR study, Tang et al. evaluated the impact of initial deployment orientation of THVs on their final orientation and neocommissural overlap with coronary arteries in 828 patients who underwent TAVR (483 SAPIEN 3, 245 Evolut, and 100 ACURATE-neo valves) from March 2016 to September 2019 at five centers.51 Co-planar fluoroscopic views were co-registered to pre-TAVR CT to determine commissural alignment. Severe overlap between neocommissural posts and coronary arteries was defined as 0–20° apart. More than 30–50% of cases had overlap with one or both coronary arteries. The investigators noted that with SAPIEN 3, despite crimping the valve at the 3, 6, 9, or 12 o’clock orientations relative to the delivery catheter, there was no impact on commissural alignment. Fortunately, the low stent frame profile of SAPIEN 3 renders commissural alignment less pertinent for coronary reaccess as wires and catheters can engage the coronary ostia above and through the top row of the stent frame. Nevertheless, coronary reaccess can be challenging in certain cases where the SAPIEN 3 stent frame extends beyond a narrow sinotubular junction.
Commissural alignment is particularly important for facilitating coronary reaccess following Evolut THV, given the long frame, relatively small stent diamonds and the supra-annular design that extends above the sinotubular junction and coronary ostia.
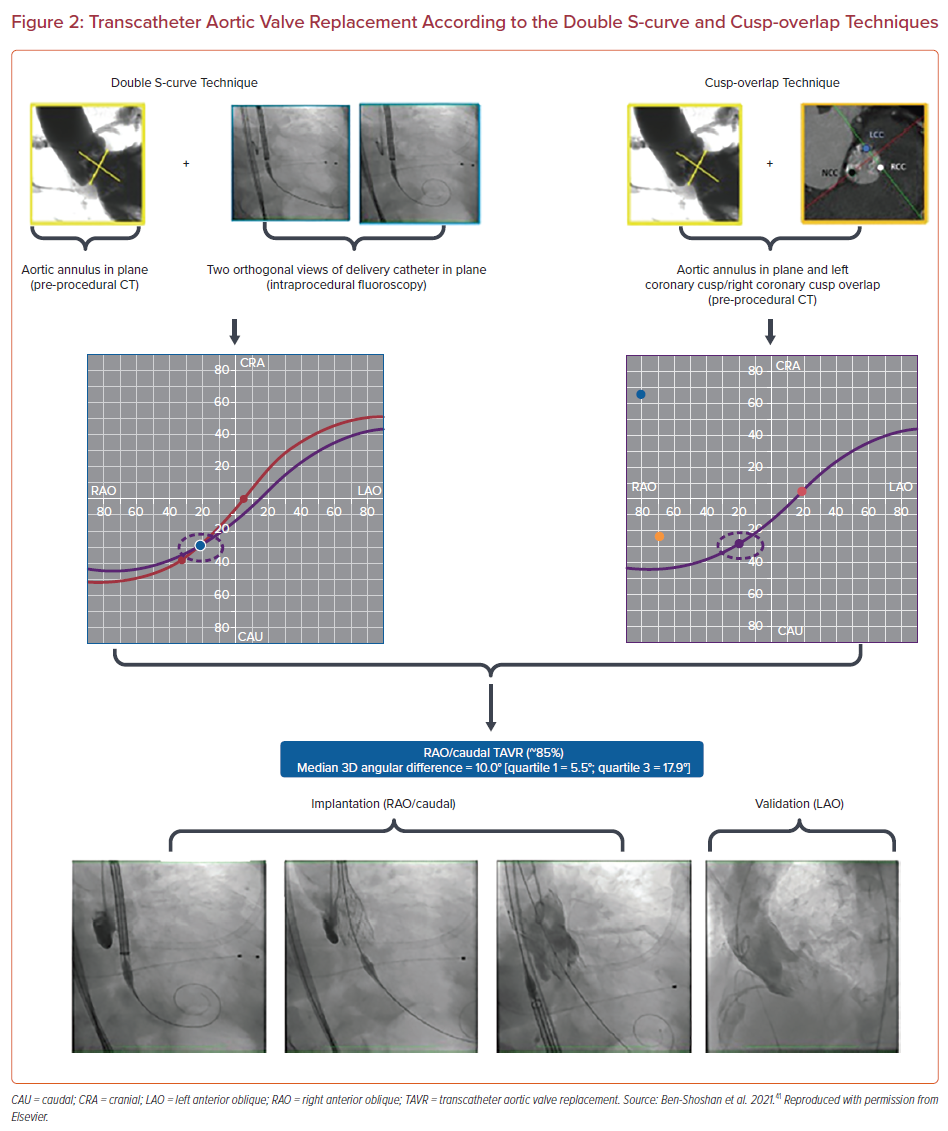
In the ALIGN-TAVR study, Tang et al. demonstrated that orienting the Evolut ‘hat’ at outer curve (OC) or center front (CF) during valve deployment resulted in improved commissural alignment and significantly less severe coronary overlap than inner curve (IC)/center back (CB) positioning with the left main artery (15.7% versus 66.0%), the right coronary artery (7.1% versus 51.1%), both coronary arteries (2.5% versus 40.4%), and one or both coronary arteries (20.2% versus 76.6%) (p<0.001 for all).51 The investigators found that having the flush port starting at 3 o’clock significantly improved the ‘hat’ position at outer curve or center front during initial deployment from 70.2% to 91.6% and reduced coronary artery overlap by 36–60% (p<0.05) (Figure 3).
The impact of the Evolut ‘hat’ marker deployment orientation on commissural alignment to determine the incidence of severe coronary artery overlap was also assessed in the Evolut Low Risk TAVR CT sub-study, where 154 of 249 patients who underwent TAVR with the conventional delivery insertion technique using the flush port at 12 o’clock were compared to 240 patients who underwent deployment using the modified technique with the flush port at 3 o’clock. The modified technique significantly improved the ‘hat’ marker orientation at OC/CF during initial deployment (93.1% versus 69.6%; p<0.001), improved commissural alignment, and reduced severe coronary overlap (left main artery: 15.2% versus 27.7%; p=0.004; right coronary artery: 11.8% versus 27.7%; p<0.001).52
CoreValve with its supra-annular design has substantial advantages in valve hemodynamic parameters, which are superior to those with SAVR, and its hemodynamic edge may also contribute to a decreased incidence of leaflet valve thrombosis compared to SAPIEN 3, although coronary access remains challenging with CoreValve compared to SAPIEN 3.6,13,53 The modified delivery system insertion technique described in the ALIGN TAVR study resulting in better commissural alignment and less coronary overlap following Evolut THV is promising and mandates further studies to validate its reproducibility.
Conclusion
Multimodality imaging plays a vital role in the accurate assessment of THV sizing and positioning, and to balance the risk of PVL against annular rupture. Prosthesis–patient mismatch post-TAVR is increasingly being recognized as being associated with higher mortality rates. SEVs have shown reduced incidence of prosthesis–patient mismatch due to their supra-annular deployment compared to BEVs. Conduction abnormalities remain a major concern following TAVR. The incidence of PPMI remains higher in TAVR compared to SAVR. An increasing volume of data suggests that low implantation depth contributes to the need for a PPM and there is now advocacy for shallow implantation of THV. Fluoroscopic cusp overlap technique in RAO/CAU projection has been demonstrated to be a reliable surrogate of the double S-curve and effectively allows an unforeshortened view of the LVOT and the delivery catheter, thereby eliminating parallax. This view offers true perception of THV depth and allows for a precise 3 mm implantation depth of Evolut bioprosthesis. Recent data suggests that SAPIEN 3 deployment in RAO/CAU projection allows higher implantation, resulting in reduced rates of PPMI. Commissural misalignment during THV deployment, particularly for the Evolut platform, is now recognized to have important implications on future coronary reaccess and aortic valve reintervention. The recently introduced technique to aim the ‘hat’ position at OC or CF of the descending aorta during initial deployment showed encouraging results to achieve better commissural alignment and reduce the incidence of coronary overlap.