Cardiogenic shock (CS), defined as a low-output cardiac state resulting in severe end-organ hypoperfusion, is a life-threatening condition requiring prompt recognition and intervention. Despite improvements in the diagnosis and management of conditions leading to CS, including early revascularization of patients with acute coronary syndrome and increased options for the application of mechanical circulatory support (MCS), the incidence of CS has increased over the past decade and in-hospital mortality remains high.1 The timely recognition, treatment, and referral of patients with CS remains paramount. However, unlike with other time-sensitive disease states, there is no coordinated management and referral network in the US for these complex cardiac patients. In this article, we summarize the evidence for timely access to cardiac shock centers, review best-practice recommendations for regionalization of care for CS patients, and summarize the advocacy and legislation work to date in the field.
Timely Access to Care for Cardiogenic Shock Patients Improves Outcomes
Significant advances over the past several decades in the care of patients with cardiovascular disease have led to overall improvements in care delivery and outcomes. For example, for patients with ST-elevation MI (STEMI), timely identification and early revascularization efforts have led to a reduction in mortality of over 20%.2 However, patients presenting with CS have not shared the same fate, and unfortunately remain at high risk of further decompensation and death. Although there is some evidence that outcomes have improved slightly in certain populations of CS patients, other studies still demonstrate mortality rates approaching 50%.3,4
Although expedient first medical contact to intervention for STEMI patients is a guideline-driven recommendation, time to intervention or initiation of treatment or support for patients with CS remains unstandardized. Once CS is suspected as the etiology for a patient’s decompensated state, timely diagnosis, treatment, and escalation of care as needed are paramount to improving survival. After the landmark SHOCK trial, early revascularization with percutaneous coronary intervention (PCI) became the standard of care initial management strategy for shock secondary to acute coronary syndrome (ACS).5 Notably, some patients with ACS are at higher risk than others for presenting with or developing CS: recent data suggest that among ACS patients, those with STEMI experience a greater in-hospital risk of CS (4.4%) than those who present with a non-STEMI (1.6%), together representing over 60,000 patients each year.6,7
For non-ACS patients with CS, there is very little randomized trial data regarding management and, as such, recommendations for this population have been extrapolated from the ACS data. Interestingly, although early revascularization efforts have decreased overall mortality in ACS patients with shock, the mortality rate for patients with ACS-derived CS remains higher than for those with a non-ACS etiology.8 Regardless, for the vast majority of patients with CS, initial management will invariably involve coronary angiography, invasive hemodynamic monitoring, initiation of vasopressor or inotropic support, and, for some, initiation of MCS. Time to intervention matters for CS: the recent FITT-STEMI trial showed that for patients with both STEMI and CS, every 10-min delay in care resulted in a 3.31% additional mortality rate.9
Some patients with CS will require MCS, although there is a notable lack of guiding data regarding who should receive support, when that support should be applied, and what type of support is best. Percutaneous options for MCS are varied. Left ventricular support devices include: the intra-aortic balloon pump, which has fallen somewhat out of favor due to lack of improved survival for CS patients in clinical trials; the Impella 2.5 and CP (Abiomed), microaxial pumps requiring intraventricular insertion across the aortic valve; and the Tandem Heart (LivaNova), which is limited by the need for transseptal puncture.10 Right ventricular support options include the Impella RP (Abiomed) and the Protek Duo (LivaNova). Finally, extracorporeal membrane oxygenation with a left ventricular vent can support the right and left ventricles and also manage hypoxia resulting from pulmonary compromise. Of these, only the Impella family of devices is approved to treat CS by the Food and Drug Administration. Whether MCS should be initiated prior to coronary intervention is also a matter of debate, with a paucity of randomized clinical trial data in this area. Data from the National Cardiovascular Data Registry show that the majority of MCS is initiated during or after PCI for patients who present with ACS.6 STEMI-DTU is an ongoing clinical trial evaluating the impact that implementation of upfront MCS (i.e. early door-to-unloading) may have on patients presenting with STEMI.11 Evidence to guide appropriate MCS application in non-ACS CS patients is even more sparse.
Limitations in evidence notwithstanding, the time-sensitive nature of CS has led to a call for a similar concept of early initiation of MCS, with a door-to-support time of ≤90 min, paralleling the STEMI literature.12 Although the field of CS as a whole is plagued by a lack of randomized controlled trials due to the complex nature of enrolling such critically ill patients, increasing retrospective evidence supports this statement. The Detroit Cardiogenic Shock Initiative evaluated the feasibility of upfront invasive hemodynamic monitoring and a protocol-driven application of early MCS with Impella for patients presenting with acute MI complicated by CS (AMICS). They found that with an average door-to-support time of 83 minutes, patients demonstrated a significantly higher survival to explant rate of 85% (versus 51% for historic institutional controls), with a survival to discharge of 76%, far above the national average.13 However, many PCI-capable centers are not able to provide access to advanced MCS options in a timely manner. In addition, for CS patients who may not require MCS but need the resources of a larger multidisciplinary team, appropriate triage of CS patients is an area of great concern. As such, how patients in CS should achieve access to appropriate care is a salient area of discussion, and an opportunity to improve the delivery of care for these critically ill patients.
Regionalization is Standard of Care for Cardiogenic Shock Patients
While access to cardiac care has increased over the past several decades, there is mounting evidence that higher-volume centers and operators are directly linked to improved outcomes. Although this has been seen across many other areas of medicine, within the realm of cardiac emergencies the evidence suggests a direct correlation between both operator and institutional volume with outcomes for PCI and coronary artery bypass grafting.14,15 This also remains true for the most high-risk subgroups: for patients presenting with acute MI (AMI), there is a significant decline in mortality if PCI is performed by experienced operators at high-volume institutions. This has also been borne out in other high-risk subgroups, such as patients with heart failure, multivessel disease, or other comorbid conditions.16 Based on these data, it is a logical conclusion that for patients with CS of all etiologies, mortality and outcomes could be improved at high-volume centers.
A recent large trial reviewing more than 500,000 admissions for CS demonstrated that large-volume centers are more likely to appropriately treat this complex patient population.17 Compared with lower-volume centers, high-volume centers were more likely to offer standard of care revascularization strategies, as well as apply more advanced MCS options. The authors also noted an increase in the application of complementary therapeutic options for patients with end-organ dysfunction, such as dialysis, at higher-volume facilities.17 Most importantly, a lower annual hospital case volume for patients with CS was associated with a significantly increased odds of in-hospital mortality. The authors’ conclusion raised the question as to whether lower-volume centers should consider early stabilization and transfer to higher-volume centers for patients with CS.17 This conclusion was supported by data from the Inova Heart and Vascular Institute’s INOVA-SHOCK Registry, which showed that timely and protocolized application of MCS for CS patients at a high-volume center significantly improved survival.18
This concept, known as regionalization of care, involves establishing systems of care whereby higher-volume, specialized facilities receive patients from outlying regional hospitals using clearly defined criteria and established transfer protocols to facilitate the timely triage of a special population of patients. This schematic has historically been successfully implemented for other emergency medical conditions, such as trauma, STEMI, and stroke, and has been associated with improved outcomes. For example, a large meta-analysis found that the establishment of a national trauma center system resulted in a 15% improvement in mortality for this high-risk patient population.19 Similarly, for cardiac emergencies, many studies have shown the feasibility and benefit of the regionalization of care for patients suffering from STEMIs, out-of-hospital cardiac arrest (OHCA), and aortic dissection.20–22
At the same time as these regionalization efforts have improved delivery of care in other realms, the standard of care for the definitive management of patients with CS has evolved. Recognizing the increasing complexity of patients with acute cardiac pathologies, various international organizations have recommended certain resource, organizational, and staffing requirements for the best delivery of cardiac critical care.23,24 This often involves a multidisciplinary shock team, staffed by interventional cardiologists, advanced heart failure cardiologists, cardiothoracic surgeons, and cardiovascular intensivists, as well as advanced support staff and ancillary services, to include emergency care and transport services. Many CS patients, especially those who require MCS, experience complications, setbacks, and the prospect of long-term care, and the spiritual or emotional needs of this patient population may differ from that of other patients with chronic medical conditions.25 Access to a palliative care team that specializes in the treatment of patients living with advanced heart failure or other end-stage cardiac conditions may only be accessible at a high-volume cardiac center. Establishing palliative care referral criteria for these patients as part of a standardized treatment algorithm may also be useful.26 Research, quality improvement processes, and education are also integral parts of improving the delivery of care to this patient population.24 This has implications for the education and training of the next generation of physicians and providers working in the cardiac space. Education regarding the management of CS, resource utilization, systems of care, and participation in the multidisciplinary heart team approach should be implemented into training pathways.
In summary, to improve the delivery of care to these complex patients, individual hospitals must come together to form partnerships within a regional referral network. Although the acuity and therapeutic capabilities of the ‘spoke’ hospitals may vary, establishing agreed-upon management strategies, delineating clear criteria for the application of advanced MCS, and acknowledging predefined triggers for consideration of transfer are the cornerstones of regionalization efforts.
Advocacy Matters: National Leaders and Associations Call for Regionalized Cardiac Shock Centers
Understanding the time-sensitive nature of access to advanced cardiac care, there is a clear need for organization of the access process: a higher level of care cannot save lives if it cannot be accessed in a timely manner. Based on the most recent US census data, >60 million Americans live in a rural setting, and it is likely that even more potential patients live a significant distance from a center capable of providing advanced cardiac care.27 A large systematic review from 2016 addressed the degree to which healthcare outcomes are tied to where patients live, finding that lengthy travel times or long distances to appropriate healthcare facilities is strongly associated with worse outcomes.28 In short, we must get the right patients to the right place in the right time.
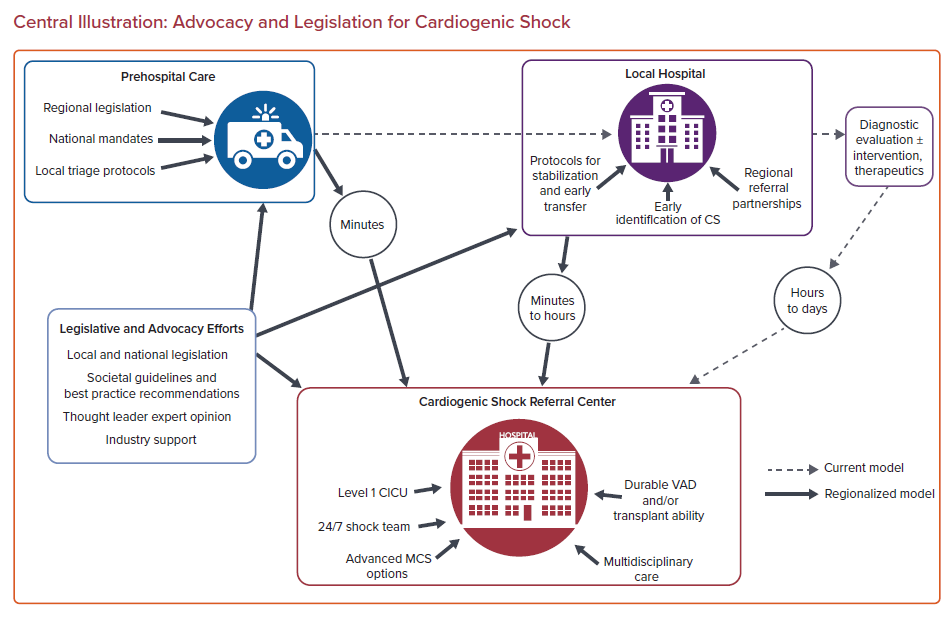
Drawing on experience from the stroke and trauma populations, it stands to reason that CS patients represent a key patient population that would benefit from regionalization of care with a hub-and-spoke schematic. Although discussed previously, this concept was formally realized in 2017, when the American Heart Association (AHA) advocated for regionalization of CS patients in a similar manner to that of STEMI and OHCA patients in a landmark scientific statement.29 This proposed system of care contained detailed requirements of the receiving ‘hub’ hospital, to include a Level 1 cardiac intensive care unit (CICU) with 24/7 access for consultations, referrals, and the application of MCS technologies. Similarly, it recognized the varying capabilities of the outlying ‘spoke’ hospitals, highlighting the importance of creating CS treatment algorithms to “standardize regional management practices, provide futility parameters, and determine the timing of transfer once the diagnosis of refractory CS is established.”29 Similar statements have echoed the staffing and capability requirements to merit a Level 1 CICU designation.20
Tchantchaleishvili et al. took this a step further, advocating for organized, statewide networks of tiered centers to care for patients with AMICS.30 In their position piece, Tchantchaleishvili et al. argue that the pillars of acute trauma management in the ‘golden hour’ should be applied to the CS patient: timely recognition of CS, stabilization, and ultimately transfer to another facility with the appropriate resources. Tchantchaleishvili et al. stress that when a patient’s needs exceed a local or initial receiving center’s resources, only essential procedures should be performed, followed by expedient transfer.30 For CS patients, this may mean PCI of a culprit lesion and initiation of inotropic or vasopressor support prior to transfer to a regional facility capable of quickly upgrading the patient to MCS if needed, with the ability to manage the entirety of that patient’s needs for the duration of their illness. Tchantchaleishvili et al. echo the AHA’s spoke-and-hub model, drawing again on the trauma verbiage to describe a level 1 center that is capable of temporary and long-term advanced cardiac care, to possibly include advanced MCS capabilities, a left ventricular assist device (LVAD) program, or a cardiac transplant program.30 Level 2 centers would be capable of temporary MCS support, and level 3 centers would have no ability to provide MCS; both of these types of center would focus on expedient stabilization and transport to level 1 centers by ground or by air.30
For their part, Rab et al. mirrored these recommendations, arguing for a similar ‘systems of care’ treatment pathway for patients with AMICS, referencing again the hub-and-spoke model.12 Rab et al. advocated for designations of cardiac shock care centers, with similar tiers based on available resources to include MCS and the presence of an organized shock team. They also highlighted the importance of the chain of survival for CS patients, addressing the need for close collaboration with emergency medical services (EMS) and local emergency departments, as well as the delivery of coordinated, best-practice care between the cardiac catheterization laboratory and the intensive care unit.12
Altogether, it is evident that thought leaders and national associations alike have achieved a consensus: regionalization of cardiac care for patients with CS represents best-practice care and has the potential to improve outcomes for a patient population that has historically seen unacceptably high mortality rates despite innovation in practice. The framework for CS patients is also clear, focusing on timely recognition, appropriate triage, and expeditious transfer to an appropriate level 1 receiving cardiac care center. How, then, can we achieve a national model for delivery of care?
Legislative and Regulatory Paths Forward for Improving Care for Cardiogenic Shock Patients
Cardiovascular disease remains the leading cause of death in the US, killing almost four times as many Americans each year than trauma.7,31 However, coordinated legislative efforts for a national cardiac care system remain limited, and are essentially absent with regard to CS patients. Historically, meaningful, systematic change in the healthcare delivery system in the US has required national and state legislative efforts. Looking at how other similar networks of care have been designed and the legislative support behind them can offer insights into how regionalized cardiac care centers can work to better serve patients with CS.
Reference is frequently made to the trauma center designations and networks of regionalized care as a potential model for the treatment of cardiac patients. Although efforts to improve the timely care given to trauma patents in the US dates back to the Civil War, dedicated efforts for a regional trauma network began in earnest in the 1960s with the publication of a seminal report by the National Academy of Sciences entitled Accidental Death and Disability: The Neglected Disease of Modern Society, which labeled trauma care a national epidemic.32,33
After the publication of that report, national surgical and trauma organizations in the US, such as the American College of Surgeons, worked to develop national standards for trauma centers, similar to how the AHA has advocated for standards as to what designates a level 1 CICU. However, for trauma patients, legislation was necessary to formalize these recommendations. Examples of such legislation include the 1966 Highway Safety Act and the 1973 Emergency Medical Services Systems Act, both of which were key in establishing pre-hospital EMS systems and transportation services to get patients to trauma centers in a timely manner. State-level regulation also played a key role: legislation regarding seat belt laws and air bags in cars, for example, was integrated into the national and state law books throughout the 1970s and 1980s, addressing the integral role that injury prevention has in the realm of trauma.32 During this time, the American College of Surgeons established the process for categorizing hospitals into different levels, reflecting the type of trauma care the hospital was able to provide. To date, although the American College of Surgeons does not officially designate a hospital as a trauma center per se, it serves an integral role in surveying hospitals and providing national-level guidance with regard to best practices.
All this advocacy and legislative effort has worked: a landmark study published in 2006 showed that the risk of death for trauma patients was significantly lower if they were treated at a level 1 trauma center, concluding that national-level regionalization efforts should remain in place.34 However, the work for improving trauma care continues; similar to the field of cardiac emergencies, to date there is still no formal national trauma system in the US, and one-third of Americans live in an area without a complete trauma system.32
Looking at cardiac emergencies, the AHA has continued to advocate for coordinated systems of care and has achieved some degree of success with programs, such as Mission: Lifeline, which seeks to support local and regional healthcare systems to improve the care they give to STEMI patients. However, the implementation of STEMI systems of care in the US is limited by regional and local barriers and varies from state to state.35 To improve networked care for trauma patients, national legislation was needed; however, most legislative efforts in the US for patients with cardiac emergencies, especially for the population of patients with CS, have remained at the state level and are regionally quite variable. For example, beginning in 2006, Washington state established a formal cardiac triage process for cardiac patients in the field in addition to stroke patients, known as the Washington State Emergency Cardiac and Stroke (ECS) System. Although almost all prehospital systems have established criteria regarding hospital triage for STEMI patients, Washington state’s protocol includes cardiac arrest patients with return of spontaneous circulation, patients with cardiac conditions leading to pulmonary edema, and those who are hypotensive.36 The Washington State ECS System protocol also prioritizes transfer to a level 1 cardiac hospital over a closer level 2 facility should time allow; these named designations are specific to Washington state, but include criteria such as 24/7 availability of the cardiac catheterization laboratory, cardiac surgery coverage, and appropriate intensive care unit services. Although that protocol does not address patients with CS specifically, this network of care represents progression from historic management of patients with cardiovascular conditions, and acknowledges the concepts of facility triage for patients in CS based on field criteria. Success in implementing this novel structure was achieved through state-level legislation, which required the Washington Department of Health to support an emergency cardiac system, establish protocols and procedures with EMS leaders, and encourage the voluntary participation of local hospitals.37
A similar system has been instituted in the state of Arizona. Led by the Arizona Department of Health Services, the Save Hearts in Arizona Registry and Education (AZ SHARE) system sought to establish formal ‘cardiac receiving centers’ with enhanced capabilities to care for patients after cardiac arrest and other cardiovascular emergencies.38 The success of this program was highlighted in a 2014 paper published in the Annals of Emergency Medicine, reporting a significant improvement in both survival and favorable neurologic outcomes for patients experiencing out of hospital cardiac arrest after the implementation of this regionalization program.39 Extrapolating this success, it stands to reason that expanding regionalized systems of care to CS patients has the potential to save additional lives.
Other states continue to advocate for this principle via legislature: in the state of Georgia, legislation passed in 2017 established the Office of Cardiac Care within the Georgia Department of Public Health, which delineates EMS triage protocols and designates levels of ‘emergency cardiac care centers’ for patients suffering from cardiac emergencies.40 However, it remains clear that there is no unified, collaborative national legislative initiative to coordinate, regionalize, and improve the systems of care for patients with cardiac emergencies, especially for those with CS. Whether the nuances of such a program should be allocated to the individual states or involve a more national legislative presence is a matter for debate. Regardless, the achievement of meaningful, longitudinal improved care for this patient population will require close collaboration with national societies, state and national legislative bodies, and thought leaders in the field of cardiovascular emergencies. Finally, financial remuneration can be used, at times, to encourage the implementation of best practices. Outcome-based metrics are increasingly common methods of indexing care and reimbursement, including via government organizations. For example, the Centers for Medicare and Medicaid requires a multidisciplinary heart team approach for patients undergoing transcatheter aortic valve replacement, with requirements as to hospital- and provider-specific volumes and experience.41 Although complex to enact, a similar approach could be implemented for certain CS patients to encourage best-practice care with ultimate referral to a tertiary or quaternary center with the appropriate immediate and long-term resources.
In a similar vein, legislative and regulatory efforts that support increased research for CS patients are needed. As mentioned above, strong evidence for best practices is limited by the inherent challenges that come with studying such a critically ill yet multidimensional population as patients with CS. This has led to the development of variable practice patterns and the use of novel MCS devices without clear guidelines as to their application. Greater leadership and coordination of regulatory efforts in this realm are needed.
The Cardiac Safety Research Consortium ThinkTank is an example of an organization that was created in an attempt to address these challenges.42 This group of leaders in the field, including those from clinical practice and industry from the US and Canada, highlighted several barriers to the generation of high-quality evidence for this patient population, including: the lack of a standardized definition of CS; the use of MCS devices for off-label populations, such as patients with CS without AMI, or the use of these devices for an unspecified duration; challenges regarding enrolling CS patients in clinical trials, including lack of consent and the heterogeneity of these patients; and the operational and logistic challenges of designing a randomized controlled trial for CS patients. They drew parallels to successful research in the stroke population, wherein heterogeneous patients with time-sensitive conditions presented for care and were enrolled with clinical and ethical success. Finally, they called for an international standard for emergency research to aid in the enrollment of CS patients in further studies. The Cardiac Safety Research Consortium ThinkTank also agreed with the establishment of regional centers for the care of the CS patient.
If parallels are drawn to the trauma systems of care as a benchmark, we are on the right track with regard to our efforts to standardize and regionalize the care we provide for patients with CS. Leading national organizations have published clear position pieces and white papers calling for greater coordination and collaboration of care. Efforts are underway to capture high-quality data in this challenging field, and we are moving towards uniform definitions of the disease process with consensus on best practices and standards of care. State legislative bodies have acknowledged gaps in systems of care for cardiac patients and are using their regulatory powers to encourage regional- and state-level collaboration. Regulatory bodies are beginning to recognize expanded indications for MCS devices, although this remains an area in need of further work. The next step involves a clear, unified vision of care across the entire US, and even internationally, with consistent verbiage, clear evidence-based guidelines for best-practice management, and definitive legislative support at the state and national levels, including funding for registry data and quality initiatives.
Conclusion
CS is a complex state of hemodynamic embarrassment that, despite improvements in treatment modalities and delivery of care, often remains challenging to diagnose, frequently fails medical management alone, and confers a high mortality rate. Timely application of advanced strategies, including MCS for some patients, is of the utmost importance for this complex and critically ill patient population. Based on data and experiences with other life-threatening conditions, a nation-wide, well-coordinated system of regionalized care for patients with CS will facilitate earlier recognition, stabilization, and transfer, with the potential to improve outcomes due to the more rapid application of appropriate escalation of support and care. The importance of establishing improved processes to obtain clinically rigorous evidence with the goal of establishing clearly defined best practices for this patient population cannot be understated. National and state leaders in the US, as well as federal regulatory bodies, physician thought leaders, industry representatives, and national organizations, must collaborate and advocate for a clear, structured cardiac shock center network with a tiered model for to deliver care to the sickest population of cardiac patients.