Aortic stenosis is one of the most common forms of acquired degenerative valvular disease and is associated with poor survival after the onset of symptoms.1 Treatment options for patients with aortic stenosis include medical therapy, surgical aortic valve replacement (SAVR) with either tissue or mechanical valves, or transcatheter aortic valve replacement (TAVR) with either balloon-expandable or self-expanding valves via either transfemoral or alternative access routes. Here, we review the lifetime management of care for young (<65 years of age), low-risk patients with severe aortic stenosis who are treated with TAVR versus SAVR. We cover how the management of aortic stenosis has evolved, review current guidelines and recommendations, and discuss special considerations for the use of TAVR versus SAVR in young, low-risk patients.
How the Management of Aortic Stenosis Has Evolved: History of TAVR
For decades, SAVR has remained the gold standard of treatment for aortic stenosis. An option for SAVR is a mechanical valve, which requires the patient to be on lifelong warfarin therapy. PROACT showed that patients with a lower target international normalized ratio (INR) of 1.5–2.0 are feasible candidates for an On-X mechanical valve (On-X Life Technologies).2 In PROACT Xa, ongoing studies are being performed to determine the potential of apixaban as the anticoagulant of choice in patients with an On-X mechanical valve.3 Dabigatran was previously explored as an option for mechanical heart valves in the RE-ALIGN trial; however, that trial was terminated prematurely due to increased rates of thromboembolic and bleeding complications in the dabigatran group.4 Other options for a mechanical valve include bioprosthetic SAVR and the Ross procedure with a pulmonary autograft.
TAVR has evolved from a niche procedure used in extreme-risk patients to an integral part of the treatment algorithm for patients with aortic stenosis. The PARTNER trials played a pivotal role in providing evidence of the safety, feasibility, and efficacy of TAVR.5 Globally, thousands of centers are now using TAVR, which has led to an expansion in the treatment of aortic stenosis. The Society of Thoracic Surgeons (STS)–American College of Cardiology (ACC) Transcatheter Valve Therapy (TVT) registry, which provides an overview of current TAVR practices in the US, showed that 276,316 patients overall, most of whom were inoperable, extreme/high risk (65%) or intermediate risk (30%), underwent TAVR in the US between 2011 and 2019, with the volume of TAVR exceeding that of SAVR in 2019.6 The registry also provides data on real-world outcomes of TAVR from 2011 to 2019, including a decrease in the 30-day mortality rate (from 7.2% to 2.5%) and stable rates of stroke (from 2.8% to 2.3%) and permanent pacemaker insertion (from 10.9% to 10.8%).
With the growth of TAVR use and the expansion of indications to low-risk patients, it is important to consider how the index procedure, whether TAVR or SAVR, affects the feasibility and optimization of a future transcatheter valve-in-valve (ViV) procedure.
Guidelines
The 2020 ACC and American Heart Association guidelines for the management of valvular heart disease include recommendations regarding valve choice for patients with severe aortic stenosis.7 According to these guidelines, patients aged <50 years should be considered for a mechanical over bioprosthetic aortic valve replacement (class 2a recommendation), or a Ross procedure if the patient prefers a bioprosthetic aortic valve (class 2b recommendation). For patients between 50 and 65 years of age, either a mechanical or bioprosthetic aortic valve replacement is recommended (class 2a recommendation). For patients aged >65 years, choosing a bioprosthetic aortic valve over a mechanical valve is considered reasonable (class 2a recommendation). For patients between 65 and 80 years of age for whom a bioprosthetic aortic valve replacement is reasonable, the heart team should consider TAVR or SAVR (class 1 recommendation), whereas for patients aged >80 years with no anatomic contraindications, TAVR should be considered (class 1 recommendation).7
The 2021 European Society of Cardiology and European Association for Cardio-Thoracic Surgery guidelines for the management of valvular heart disease also include recommendations regarding the use of SAVR versus TAVR, with SAVR being recommended for younger patients with a low risk of surgery (age <75 years and STS Predicted Risk of Mortality [PROM]/EuroSCORE II <4%) or for those who are operable and unsuitable for transfemoral TAVR (class 1 recommendation).8 TAVR is recommended for older patients (age ≥75 years) or for those who are high risk (STS-PROM/EuroSCORE II >8%) or unsuitable for surgery (class 1 recommendation). For all other patients, the recommendation of SAVR or TAVR treatment depends on clinical, anatomical, and procedural factors, which should be discussed by a heart team (class 1 recommendation).8
Issues and inconsistencies among the guidelines have been widely discussed, particularly regarding the lack of evidence for using age cut-offs to help determine the choice of intervention for treating aortic stenosis.9,10 In randomized controlled trials comparing SAVR and TAVR, the choice of intervention was made on the basis of estimated risk, not age. In addition, the cardiovascular community has echoed caution in recommending TAVR to young, low-risk patients given the lack of data on intermediate- or long-term outcomes in this patient population and the unique issues related to TAVR compared with SAVR.11 Certainly, the choice between SAVR, TAVR, and other valvular options in the treatment of young, low-risk patients with severe aortic stenosis requires not only a heart team approach, but also a patient-centered discussion regarding the patient’s wishes. In addition, despite a surge in the number of TAVR procedures, SAVR remains indicated in patients with specific conditions, such as bicuspid aortic valve disease, aortic regurgitation, and endocarditis, as well as in patients with concomitant cardiac conditions warranting surgery (e.g. coronary artery bypass grafting, multivalvular disease, ascending aneurysm repair), patients with failed TAVR or emergency conversion, or patients who are not candidates for TAVR.
Issues with TAVR in Young, Low-risk Patients
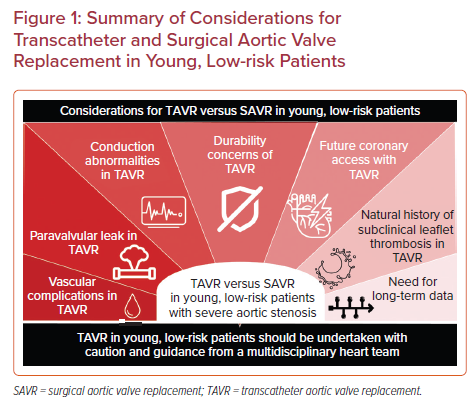
Evidence from many randomized clinical trials, prospective studies, and the observational STS-ACC TVT Registry has shown that, compared with SAVR, TAVR has acceptable clinical outcomes in low-risk patients.12–17 In these studies, the mean or median patient age was between 68 and 79 years. All-cause mortality was lower in TAVR than SAVR groups at 30 days and 1 and 2 years, although the study designs of the low-risk trials, especially with respect to the composite endpoints and time frames, have remained controversial. Furthermore, outcomes in these low-risk trials were examined in carefully selected patient groups that are unlikely to represent the real-world patient population. For example, the percentage of patients excluded from randomization was 15% in the Evolut Low-Risk trial and 34% in the PARTNER 3 trial.12,13 In addition, several issues with TAVR in young, low-risk patients need to be addressed (Figure 1).
First, vascular complications remain the primary safety concern with TAVR.18,19 Major vascular complications were reported in 12% of patients who underwent first-generation TAVR.20 Over time, the rate of major vascular complications has decreased significantly to 1.3% (from 5.6%) in recent trials and to only 0.7% in the low-risk cohort of the STS-ACC TVT Registry.12,13,17,21,22 Vascular complications are more prevalent in higher-risk patients, are associated with longer hospital length of stay and increased adverse outcomes, and pose significant challenges for future interventions.23–25
Patients with a bicuspid aortic valve (BAV), especially Sievers type 0, have been underrepresented in previous trials and studies.12,13,16,17,22 The Low Risk Bicuspid trial and Evolut Low Risk Bicuspid trial showed acceptable outcomes with TAVR in patients with BAVs; however, a higher rate of paravalvular leak (PVL) remains a concern.17,26 Excessive leaflet calcification and moderate raphe calcification have also been linked to PVL and worse outcomes in patients with BAV.27 Although significant PVL is less common in newer than older valve generations, mild PVL is frequently reported in patients with TAVR (35% in the Low Risk Bicuspid trial, 40% in the Evolut Low Risk Bicuspid trial).16,28 Because the long-term effects of paravalvular leak in younger patients are unknown, these prognostic factors need to be considered in the evaluation of young patients with BAVs.
In contrast with the decades of long-term durability data available for SAVR, no long-term durability data exist for TAVR. In young, low-risk patients, long-term survival and valve durability outcomes are paramount. Long-term data from the PARTNER 2 trial showed a trend towards a lower rate of all-cause death/disabling stroke in the SAVR group.24 Similarly, although the TAVR group had fewer primary endpoint events than the SAVR group at the 2-year follow-up, the TAVR group had more deaths, strokes, and valve thrombosis events from 1 to 2 years.29 In addition, a lack of consistent commissural alignment with TAVR may affect durability as well as future coronary access and redo TAVR.30
A more important issue with TAVR durability has arisen in the context of failed TAVR. Currently, there are two strategies for managing failed TAVR: ViV TAVR or surgical explantation of the TAVR, followed by implantation of a new prosthesis. Although ViV TAVR is less invasive, coronary obstruction is a concern, especially in patients who receive a self-expanding transcatheter heart valve (THV) due to the supra-annular leaflet position. Surgical explantation of the implanted THV can be technically challenging, with high operative mortality rates and high observed-to-expected 30-day mortality ratios.31 Aortic root and ascending aorta reconstructions are often required, especially with self-expanding transcatheter valves.31 These issues should be taken into consideration when evaluating the options for young patients who may need multiple interventions within their lifetime.
TAVR-related conduction abnormalities remain a common complication of this procedure. These conduction disturbances primarily present as new-onset left bundle branch block (LBBB) or high-grade atrioventricular block requiring permanent pacemaker (PPM) insertion. New-onset LBBB was associated with a greater risk of PPM insertion and 1-year cardiac mortality, as well as a tendency towards increased all-cause mortality.32 Furthermore, PPM insertion rates were consistently higher in patients who underwent TAVR than in their SAVR counterparts.12,13,22 PPM insertion is linked to increased rates of subsequent heart failure, rehospitalization, and mortality.33 Further studies with longer follow-up times are needed to confirm the detrimental effects of long-term pacing among TAVR recipients. Strategies to reduce the risk of these complications and to improve how they are managed are essential, especially as TAVR indications are expanding to lower-risk patients.
Future coronary access after TAVR is a concern in low-risk patients. Previous low-risk trials have shown that coronary artery disease is prevalent in more than 20% of patients with severe aortic stenosis.12,22 Obstruction by displaced aortic valve leaflets, transcatheter valve stents, or commissural suture posts could impede coronary access after TAVR in young patients with existing coronary artery disease or in those who may develop it later. Although the commissure alignment technique improves the rate of successful coronary access after TAVR with supra-annular THVs, aligned supra-annular THVs carry a higher risk of unfeasible/non-selective coronary access than do intra-annular THVs.34 Other factors, such as low sinus of Valsalva and a high THV–sinus of Valsalva relationship, were found to be predictive of impaired coronary access after TAVR.35 These technical and anatomical features are important for the heart team to consider when making decisions regarding therapeutic and prosthesis selection in younger patients with a longer life expectancy.
Finally, an increased incidence of subclinical leaflet thrombosis has been shown in TAVR patients.36 A large, real-world cohort study by Garcia et al. showed that subclinical leaflet thrombosis was independently associated with long-term mortality.37 Further studies are needed to evaluate the effect of oral anticoagulants on subclinical leaflet thrombosis and the association of oral anticoagulants with clinical outcomes.
Transcatheter Versus Surgical Aortic Valve Replacement in Young, Low-risk Patients: Where to Go From Here
The development of TAVR has opened up more options for the treatment of patients with severe aortic stenosis. Although TAVR was initially limited to inoperable and high-risk patients, the indications for TAVR have been extended to intermediate- and low-risk patients. According to the current American Heart Association guidelines, shared decision making is recommended in patients aged ≥65 years to determine whether SAVR or transcatheter valve implantation (TAVI) is more appropriate based on the balance of patient longevity and valve durability.38 With the exception of the Low Risk TAVI (LRT) bicuspid prospective study, randomized clinical trials and other prospective studies evaluating TAVI in low-risk patients reported a mean age between 70 and 79 years.12,13,16,17,22 Whether these data can be extrapolated to low-risk patients as young as 65 years of age remains unknown. In addition, patients with BAVs were excluded or underrepresented in all the major trials and prospective studies, further emphasizing that the trial findings may not be generalizable to the whole patient population. Extrapolation of the trial data to younger patients should be done with caution.
As TAVR is being offered to younger patients, many may opt for this less invasive approach. However, data for this age group with respect to long-term survival and prosthetic durability are currently lacking for TAVR. Strategies for the management of aortic valve disease in young patients should be discussed within the heart team, while also considering individual patient anatomy and lifetime expectations. More importantly, the initial therapeutic decision significantly affects future therapeutic options, and should be evaluated carefully and tailored to individual patients.34
The management of aortic valve disease will continue to evolve. Newer tissue valves such as RESILIA valves (Edwards Lifesciences) could be game changing. RESILIA tissue valves have been proposed to protect valve leaflets against calcification, with a demonstrated safety profile with 5 years of follow-up.39–42 Furthermore, these valves are built with an expandable valve frame that can facilitate future ViV procedures.43 With respect to mechanical prostheses, lower-intensity mechanical valves could be revolutionary. PROACT showed comparable efficacy and a better safety profile in young patients with an On-X valve and a lower INR target of 1.5–2.2 The ongoing PROACT Xa will evaluate apixaban as another alternative to warfarin in patients with an On-X valve.3 Recently, the cardiovascular community has shown renewed interest in the Ross procedure. Several studies have shown that the Ross procedure can be performed safely and reproducibly in selected patient populations.44–46 More importantly, the Ross procedure is associated with better long-term outcomes than conventional aortic valve replacement, especially in younger patients with an active lifestyle and long life expectancy.47 These newer surgical approaches should be discussed with patients during heart team discussions.
Conclusion
In managing the lifetime care of young, low-risk patients who have aortic valve disease with severe aortic stenosis, it is important to bear in mind current evidence and special considerations for TAVR versus SAVR. Ultimately, until ongoing clinical trials with long-term follow-up data shed light on whether interventions for aortic stenosis can be broadened to a low-risk population, TAVR in young, low-risk patients should be undertaken with caution and with guidance from a multidisciplinary heart team.










