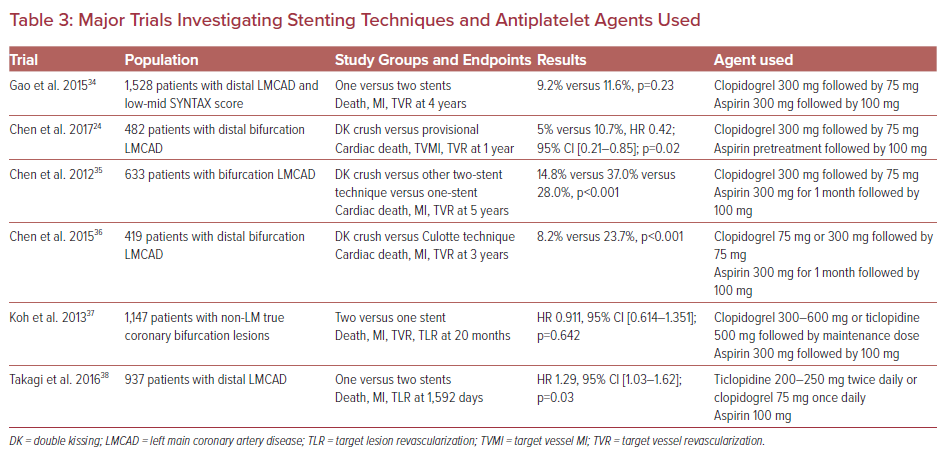
Left main (LM) coronary artery disease (CAD) is a potentially fatal condition with poor prognosis as a result of a large myocardial territory at risk.1 Therefore, diagnosis, assessment, and treatment of LM-CAD are crucial to decrease the risk of future events.2,3 It is now well established that revascularization is recommended for any LM stenosis ≥50%, regardless of symptoms, ischemic burden, or concomitant CAD. The type of revascularization – coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) – depends on many factors, namely predicted surgical mortality, anatomical complexity of CAD, anticipated completeness of revascularization, patient comorbidities, and other technical aspects (Figure 1).4
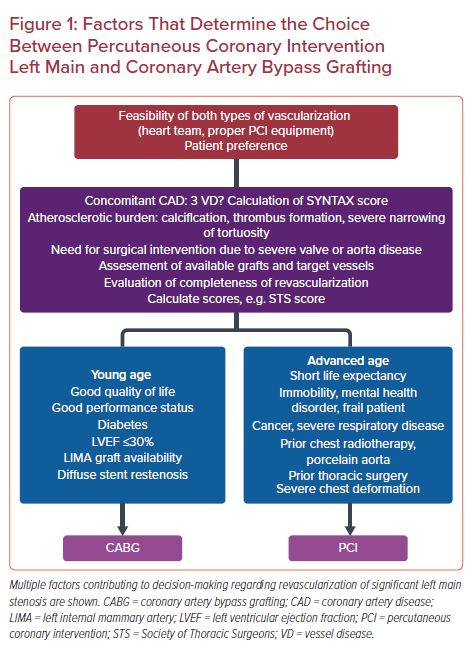
PCI of the LM shaft is a procedure that has been associated with better clinical outcomes and a lower need for late repeat revascularization than PCI of the distal bifurcation, which is generally considered more challenging.5 The latter can partially explain why, until recently, some authors did not define PCI of the LM as a complex procedure, in contrast to the recently published European guidelines.6–8 Conversely, bifurcation PCI is considered complex when at least two stents are implanted according to the European Society of Cardiology focused update on dual antiplatelet therapy (DAPT).7 Nevertheless, there are studies where stenting bifurcation lesions with side branch diameter ≥2.5 mm is also considered of increased complexity, whereas in the DEFINITION study, the authors defined complex bifurcation lesions by high-risk angiographic features, as shown in Table 1.4,9
In the context of these high ischemic risk interventions, there is a growing interest regarding the optimal antithrombotic treatment type and duration, with decision-making based on the clinical presentation, the baseline risk of bleeding, and thrombotic events, as well as the chosen stenting strategy.
PCI Versus CABG for Left Main Coronary Artery Disease
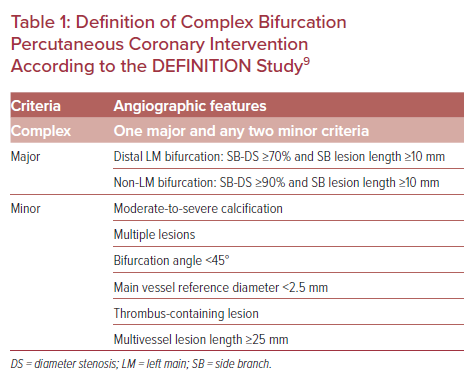
Until recently, CABG was considered the most appropriate treatment of LM-CAD; however, recent knowledge suggests that PCI can be an alternative to CABG in specific subgroups of patients with lesser extent of atherosclerotic burden.10 There are six randomized control trials comparing PCI with CABG, but only two of them – EXCEL and NOBLE – were conducted in the era of second-generation stents.11,12 All but the NOBLE trial showed that PCI was non-inferior to CABG regarding major adverse cardiovascular events (MACE), while the rate of repeat revascularization was significantly higher in the PCI arm (Table 2).13–18 Therefore, a meta-analysis of five randomized clinical trials performed by Ahmad et al. showed no significant difference in all-cause death, cardiac mortality, stroke, or MI within a mean follow-up of 67.1 months. Unplanned revascularization was less common after CABG.19
In the light of the above findings, the European Society of Cardiology suggests PCI as an equivalent to CABG regarding mortality, MI, and stroke in patients with LM-CAD and low SYNTAX score (0–22), while PCI is not favored in more anatomically complex lesions (SYNTAX score 23–32) and is contraindicated when the SYNTAX score exceeds 32.4 Regarding US guidelines, PCI is considered with a class IIa recommendation as an alternative to CABG if the coronary anatomy is associated with a low risk of procedural complications and a high likelihood of good long-term outcome (e.g. SYNTAX score ≤22, ostial or trunk LM-CAD), and the clinical characteristics predict a significantly increased risk of adverse surgical outcomes (e.g. Society of Thoracic Surgeons-predicted risk of operative mortality ≥5%).20
Overall, percutaneous revascularization of the LM demands an experienced operator, adequate preparation, and frequent use of newer techniques to offer a reliable alternative to CABG in patients with appropriate coronary anatomy.
Increased Thrombotic Risk of Left Main and Bifurcation Coronary Disease
Complex PCI is generally associated with an increased risk of MACE and stent thrombosis (ST). PCI to unprotected LM-CAD is a demanding intervention that can occasionally lead to compromised final flow or side branch occlusion.3 From an autopsy registry regarding LM PCI, the most common cause of ST was malapposition, followed by uncovered strut, multiple stent techniques, and provisional stent technique without side branch dilatation.21 Stent deposition-related factors, such as malapposition or underexpansion, by delaying endothelization and enhancing the pro-thrombotic environment, are common triggers of adverse ischemic events.22 The latter has been associated with the chosen PCI technique performed, with the two-stent technique yielding numerically better results regarding cardiac death in patients with LM bifurcation disease compared with the one-stent technique.23 Moreover, the double-kissing (DK) crush technique compared with provisional stenting has been related with better outcomes, including a significantly lower composite endpoint of target lesion failure (TLF) and ST.24 Thus, proper planning of the procedure is pivotal in bifurcation LM-CAD, as the ‘bail-out’ placement of a second stent has been associated with a higher risk of ST than planned double stenting.25
However, stenting all types of bifurcation lesions has been widely recognized as a predisposing factor for thrombosis. Among all the spectrum of complex PCI procedures, bifurcation has been directly and more prominently associated with increased risk of ischemic outcomes, including coronary thrombotic events, ST, and MI within a 2-year follow-up.26 Interestingly, implantation of two stents was associated with an even higher incidence of ST in the ADAPT-DES study (2.8% at 2-year follow-up), as well as with a higher proportion of TLF in the recently published post-hoc subanalysis of the e-Ultimaster registry (6.2% at 1-year follow-up).27,28 Of all bifurcations in the coronary circulation, those involving the left anterior descending artery and diagonal branches are the most frequently encountered in clinical trials.9
Regarding the background of thrombosis, anatomical factors, such as the fractal geometry of vascular bifurcations, increase the incidence of strut malapposition and stent underexpansion, leading to an increased ischemic risk. Except from excessive lesion calcification and inflammatory reaction, coronary artery bifurcation lesions exhibit localized turbulent flow and enhanced propensity for platelet aggregation, plaque rupture, and atherothrombosis.29 Of note, flow conditions vary in bifurcation lesion types according to the clinically oriented Medina classification, with Medina 1,0,1 more prone to atherosclerosis progression.30 In addition, shear stress plays an important role in the initiation and proliferation of coronary atherosclerosis. A baseline low shear stress after bifurcation PCI, which decreases to its minimum value post-procedural, but eventually recovers to around its baseline level, could be the setting of in-stent restenosis.31 Last, but not least, stenting LM or bifurcation lesions usually require the use of a greater total stent length or higher volume of intravenous contrast, factors that could augment the thrombotic risk. Therefore, the implementation of individualized antithrombotic regimens to mitigate the ischemic risk, according to procedural complexity, is of utmost importance.
Stenting Techniques for Left Main Bifurcation and Antiplatelet Therapy Used in Major Trials
Regarding percutaneous LM bifurcation treatment options, the European Bifurcation Club recommends a provisional side branch approach in most cases of distal disease.32 However, two-stent approaches, but mostly the DK crush technique, although technically challenging, have emerged as a preferred approach for true distal LM bifurcation lesions.33 Several observational studies and two randomized trials have already been published, while others are ongoing and the results are awaited with great interest (Table 3).3,24,34–38
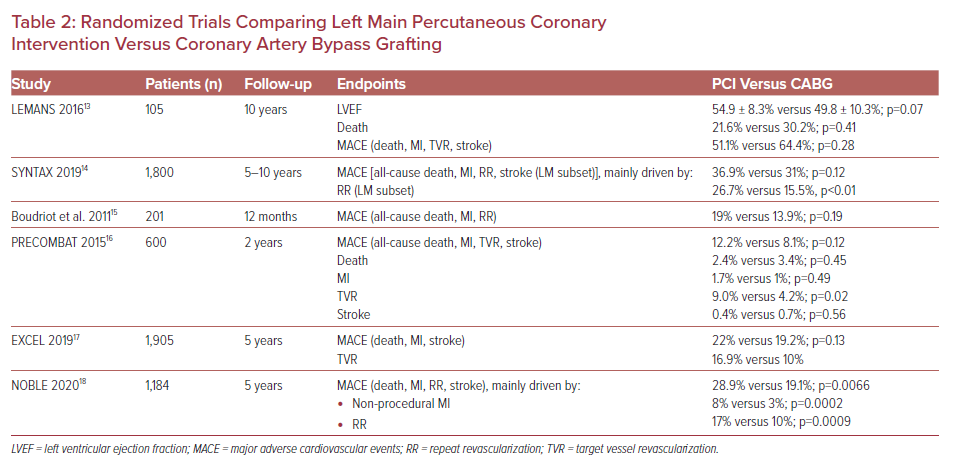
Stenting LM bifurcation is feasible with one- or two-stent techniques. A large single-center study that enrolled patients with distal unprotected LM-CAD and a low-intermediate SYNTAX score found that the two techniques did not differ significantly regarding MACE at a mean follow-up of 4 years.34 Additionally, data collected from the Korea Coronary Bifurcation Stent registry found non-inferiority of the one-stent technique compared with the two-stent technique in patients with non-LM true coronary bifurcation disease.37 Of interest, the two-stent technique has been linked to a higher incidence of TLF, mainly caused by restenosis at the ostial left circumflex artery.38 In contrast, there is cumulative evidence that the DK crush technique could be a favorable approach to true LM bifurcation PCI. The DK crush technique resulted in lower rates of a composite primary endpoint at 1-year follow-up compared with provisional stenting in patients with distal bifurcation LM-CAD.24 Moreover, the DK crush technique was associated with significantly lower rates of MACE and ST compared with the Culotte technique, mainly due to a decrease in MI (3.4 versus 8.2%, p=0.037) and target vessel revascularization (5.8 versus 18.8%, p<0.001), as well as significantly decreased rates of MACE at 5-year follow-up, compared with the other two-stent approaches or the one-stent approach, mainly driven by a reduction in target vessel revascularization (7.7 versus 30.5 versus 18.1%, p<0.001).35,36 Regarding antiplatelet agents of choice, clopidogrel at a loading dose of 300 mg and aspirin were administered in the majority of trials delineating stenting techniques (Table 2). Ticlopidine was another option, while potent P2Y12 inhibitors, such as prasugrel or ticagrelor, were not used in these studies’ populations. The duration of DAPT was mostly for 12 months.
Duration and Type of Antiplatelet Therapy in Left Main and/or Bifurcation PCI
The aforementioned findings point to the fact that patients undergoing PCI of LM bifurcation are at increased risk for thrombotic complications. Therefore, intensification of antithrombotic pharmacotherapy might be beneficial towards ischemic risk mitigation in this subset of population, although data are scarce. In trials exploring the optimal antiplatelet treatment strategy in complex PCI patients, prolongation of DAPT seemed beneficial in terms of ischemic outcomes, with the caveat of a potential higher bleeding risk.
In patients undergoing complex PCI, the prolonged DAPT (≥12 months) group experienced a significant MACE reduction in comparison with short DAPT (3–6 months) group (adjusted HR 0.56; 95% CI [0.35–0.89]), although this was associated with a higher incidence of major bleeding (1.03 versus 0.52%; incidence rate difference 0.51%). The beneficial effect of long-term DAPT on MACE reduction was homogenous between the subsets of complex PCI; that is, patients with multivessel PCI and bifurcation PCI with two stents (incidence rate difference −8.31% and −0.42% for long-term versus short-term DAPT, respectively) were favored for this approach.26 Similarly, prolonged DAPT (≥12 months) was associated with a reduced incidence of all-cause death or MI and ST at 4-year follow-up compared with a short-term strategy (<12 months) among 2,082 patients undergoing bifurcation stenting (adjusted HR 0.21; 95% CI [0.13–0.35]; p<0.001; and adjusted HR 0.08; 95% CI [0.01–0.43]; p=0.003, respectively). Interestingly, the beneficial role of prolonged DAPT was not significantly affected by lesion location (LM bifurcation) or stenting technique (one or two-stent technique).39
However, in a study by Rhee et al., extension of DAPT (≥12 months) seemed more beneficial to patients undergoing LM bifurcation PCI with implantation of two stents, as the selection of a two-stent strategy was related with higher rates of both TLF and thrombotic adverse cardiovascular events compared with a one-stent strategy in the subgroup of short-term DAPT (<12 months; HR 11.45; 95% CI [2.73–47.95]; p=0.001 for TLF; and HR 7.84; 95% CI [2.11–29.22]; p=0.002 for thrombotic adverse cardiovascular events).40
Similar conclusions regarding the optimal DAPT duration can be drawn from the multicenter Euro Bifurcation Club – P2BiTO – registry. In a 2-year follow-up period, an increased incidence of MACE was observed in the short-term DAPT group (<6 months) compared with the long-term (>12 months) and the intermediate groups (6–12 months; 14% versus 10% and 10% respectively, HR 0.72; 95% CI [0.64–0.82]; p<0.001). The difference in results remained unchanged after adjustment for clinical and angiographic characteristics (HR 0.66; 95% CI [0.58–0.77]; p<0.001). MACE-free survival was significantly lower in the group of DAPT duration <6 months (log-rank 29.5, p<0.001).41 Furthermore, results from the PRODIGY trial showed that 24-month DAPT was associated with lower occurrence of ST compared with the 6-month arm only in patients with LM or proximal LAD disease (2.8 versus 5.6%; HR 0.45; 95% CI [0.23–0.89]; p=0.02), but not in patients with other lesions, with a highly significant interaction testing (p for interaction=0.002).42
In a different approach, triple antiplatelet therapy by adding cilostazol to DAPT provided no additional long-term benefit in real-world patients undergoing bifurcation PCI. Patients on cilostazol had more comorbidities; however, no statistically significant difference was observed regarding TLF (adjusted HR 0.86; 95% CI [0.53–1.39]; p=0.53) or other outcomes (death, cardiac death, MI, ST, TVR, or cerebrovascular accident).43
Novel P2Y12 receptor inhibitors provide more potent and consistent antiplatelet action, and may be useful in higher thrombotic burden procedures, including bifurcation PCI, at the expense of higher bleeding risk. In this context, there is a tendency in favor of ticagrelor and prasugrel over clopidogrel in patients with both high ischemic and low bleeding risk, although such an approach has not been established. D’Ascenzo et al. investigated the clinical outcomes following PCI with implantation of thin stents (<100 microns) in unprotected LM stenosis or main coronary bifurcation lesions in relation to DAPT duration. The primary endpoint included cardiovascular death, MI, TVR, and ST. Clopidogrel was the most common antiplatelet prescribed. Ticagrelor was administered in 31.3% of the total study population, while prasugrel was administered in 6.2% of the total study population. At a mean follow-up of 12.8 months, MACE occurred significantly more often in the short DAPT arm of ≤3 months, compared with the 3–12-month and 12-month groups (9.4 versus 4.0 versus 7.2%; p<0.001), a difference mainly driven by MI (4.4 versus 1.5 versus 3%; p<0.001) and overall ST (4.3 versus 1.5 versus 1.8%, p<0.001). Of note, in contrast to provisional stenting, a two-stent strategy was an independent predictor of MACE (OR 1.6; 95% CI [1.1–2.3]) at multivariate analysis and ST (OR 3.241; 95% CI [1.048–10.026]) after DAPT cessation. Regarding antiplatelets, the risk of ST was significantly higher in the 3-month group irrespective of the regimen used (clopidogrel, prasugrel, or ticagrelor).44
In contrast, the results from a smaller single-center retrospective cohort study argue in favor of ticagrelor as part of DAPT during the first year of post-bifurcation PCI. Among 584 patients treated for bifurcation lesions, a higher incidence of MACE was observed in patients on DAPT with clopidogrel (34/283 patients; 12.01%) in comparison with ticagrelor (22/270; 8.15%) with an adjusted HR of 0.488 (95% CI [0.277–0.861]; p=0.013) in favor of ticagrelor. Of interest, patients treated with ticagrelor tended to have a higher proportion of true bifurcation lesions, LM disease, and higher SYNTAX score. In contrast, clopidogrel was more commonly used in patients with LAD/diagonal PCI. As expected, ticagrelor was associated with a higher incidence of total bleeding events (adjusted HR 1.791; 95% CI [1.214–2.644], p=0.003), although major bleeding rates were similar between the two DAPT strategies (2.47% and 2.96% for the clopidogrel and ticagrelor groups, respectively; adjusted HR 0.972; 95% CI [0.321–2.941]; p=0.960).45
PROMETHEUS was an observational study that compared clopidogrel versus prasugrel in patients undergoing PCI in the setting of an acute coronary syndrome (ACS). The study population was divided into a non-complex or complex PCI group; the latter including stenting LM (6.9%) or bifurcation lesions (20.1%). Prasugrel was used in almost one-fifth of the patients. Of note, its use declined with rising PCI complexity. In general, 1-year MACE rates were significantly higher in the complex PCI arm. Compared with clopidogrel, prasugrel reduced the adjusted risk for 1-year MACE in the complex PCI group (HR 0.79; 95% CI [0.68–0.92]), but not in the non-complex PCI group (HR 0.91; 95% CI [0.77–1.08]), albeit there was no evidence of interaction (p interaction=0.281).46 Of note, bifurcation PCI was one of the main parameters associated with a greater absolute risk reduction of ST (3.2%) in the prasugrel arm compared with the clopidogrel arm in the TRITON-TIMI trial.47
In the context of the GLOBAL LEADERS trial, Kogame et al. compared the experimental treatment strategy of 1-month DAPT with ticagrelor and aspirin followed by 23 months of ticagrelor monotherapy with the standard antiplatelet treatment (12 months of DAPT followed by aspirin monotherapy), focusing on patients undergoing bifurcation PCI. The latter group presented similar incidence of the primary endpoint (all-cause death or new Q wave MI) compared with undergoing non-bifurcation PCI (4.7 versus 4.0%, p=0.083), although bifurcation stenting was related with an increased need for revascularization (11.21 versus 9.19; HR 1.24; 95% CI [1.09–1.41]; p=0.001). However, the experimental treatment failed to provide a significant benefit in terms of ischemic outcomes, while the presence or absence of a bifurcation lesion did not seem to impact on its effect regarding the primary endpoint (bifurcation: HR 0.74; 95% CI [0.51–1.07]; non-bifurcation: HR 0.90; 95% CI [0.76–1.07], p for interaction=0.343).48
Finally, in the TWILIGHT-COMPLEX study, patients were randomized to ticagrelor monotherapy or DAPT after 3 months of index MI. LM PCI and bifurcation with two stents implanted represented 15.1% and 21.4% of complex PCI patients (2,342 patients in total). Among the whole spectrum of patients undergoing complex PCI, ticagrelor monotherapy compared with ticagrelor plus aspirin resulted in lower rates of the primary endpoint of BARC type 2, 3, or 5 bleeding (4.2% versus 7.7%; absolute risk difference 3.5%; HR 0.54; 95% CI [0.38–0.76]) and BARC type 3 or 5 bleeding (1.1% versus 2.6%; absolute risk difference 1.5%; HR 0.41; 95% CI [0.21–0.80]), without compromising efficacy (3.8% versus 4.9%; HR 0.77; 95% CI [0.52–1.15] for the composite of death, MI, or stroke; and 0.4% versus 0.8%; HR 0.56; 95% CI [0.19–1.67] for ST).49
The aforementioned trials highlight that the extension of DAPT beyond 12 months seems an appealing strategy toward reduction of thrombotic risk in this subset of patients provided a careful consideration of individual bleeding tendency. Regarding the antiplatelet agent of choice, there are some data supporting the use of more potent P2Y12 inhibitors instead of clopidogrel, although more evidence is required. However, longer DAPT (12–24 months) or use of novel P2Y12 inhibitors seems more reasonable for patients with bifurcation disease treated with a two-stent technique.26,40,44,45 Recently, Zimarino et al. proposed an algorithm regarding antithrombotic treatment in patients undergoing bifurcation PCI. Clinical presentation, baseline bleeding risk, stenting strategy, and possible use of intracoronary imaging are among the factors that should be taken into account for decision-making in this high-risk population.29
High-bleeding-risk Patients
Although assessment of bleeding risk is a challenging task, it seems to significantly affect decision-making regarding the type and duration of antiplatelet therapy, even in patients undergoing PCI for LM/bifurcation disease, considered to be at higher ischemic risk. Validated scores estimating bleeding risk, such as the PRECISE-DAPT and PARIS score, can be used to tailor the treatment strategy, whereas a consensus document from the Academic Research Consortium for High Bleeding Risk (HBR) defined major and minor criteria to characterize a HBR patient undergoing PCI.50–52 Recently, two prognostic models were developed to evaluate the trade-off between high thrombotic and bleeding risk, aiding toward a more personalized treatment approach.53 Interestingly, in a study by Costa et al., HBR patients (defined as PRECISE-DAPT ≥25) undergoing a complex procedure, including bifurcation stenting, did not seem to benefit from long-term DAPT (12 or 24 months) in terms of ischemic or mortality risk reduction, a fact highlighting that in cases of both elevated ischemic and hemorrhagic risk, the latter should primarily guide treatment strategy. Consequently, a DAPT of shorter duration (3 or 6 months) could be appropriate, providing careful consideration of individual clinical and angiographic factors.54
Furthermore, recent evidence supports an even shortened DAPT duration (1–3 months) with newer-generation drug-eluting stents, such as Synergy (Boston Scientific) bioabsorbable polymer-coated everolimus-eluting stent or Resolute Onyx (Medtronic) zotarolimus-eluting stent, in the HBR population, without compromising efficacy.55,56 Of note, in the Onyx One study comparing Resolute Onyx with a polymer-free umirolimus-coated stent, the former was not inferior regarding safety and effectiveness in HBR patients receiving single antiplatelet therapy after 1 month of DAPT; notably, the majority of patients were treated for complex type B2/C lesions, while up to 16% of total patients had bifurcation PCI.57 Regarding12 inhibitors have been associated with a higher incidence of bleeding complications compared with clopidogrel; thus, their role in this subset of patients is limited.7,58
Perspectives
Both European and US guidelines include bifurcation with two stents implanted as a high-risk feature for ischemic events, among other characteristics of PCI complexity, suggesting that a longer duration of DAPT (≥ 6 months) may be considered in patients with chronic coronary syndromes.7,33,59 In recently published guidelines regarding ACS, a prolonged DAPT duration (≥12 months) is a class IIa recommendation in patients without increased risk of major or life-threatening bleeding.8 Regarding the choice of antiplatelet agent in the setting of ACS, ticagrelor or prasugrel are recommended over clopidogrel due to their proven superior efficacy with a class I recommendation.7 In contrast, clopidogrel is still the preferred agent in patients with chronic coronary syndrome and PCI, while potent inhibitors may be considered with a class IIb recommendation at least as initial therapy, in specific high-risk situations of elective stenting, such as complex left main PCI or multivessel stenting.60 Interestingly, among patients who underwent complex PCI, a regimen of ticagrelor monotherapy (after an initial 3 months of DAPT with ticagrelor and aspirin) was associated with significantly lower clinically relevant bleeding rates without increasing the risk of ischemic events in comparison with the continuation of DAPT, providing an alternative strategy to double therapy extension.49
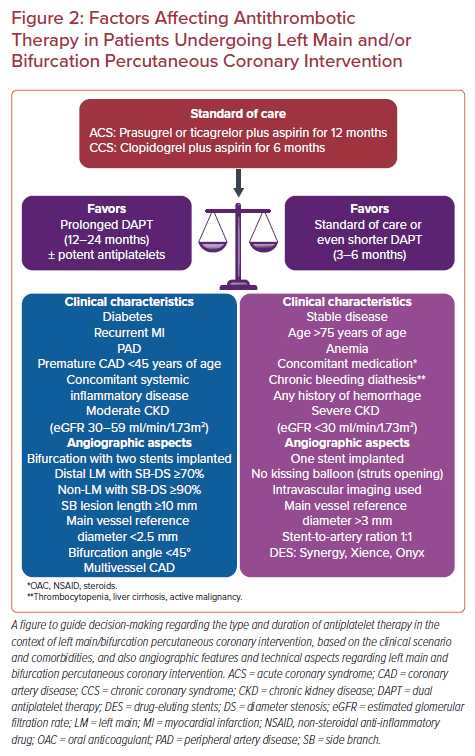
Regarding intravenous antiplatelet agents, experts suggest that patients, not pretreated with oral P2Y12 inhibitors, undergoing complex PCI could be ideal candidates for cangrelor administration during index MI.61 Glycoprotein IIb/IIIa inhibitors may still play an important therapeutic role in challenging clinical scenarios, such as revascularization of LM bifurcation, with the caveat of increased bleeding complications. Of note, the use of intracoronary bolus tirofiban may represent an effective and safe strategy to achieve rapid thrombus resolution in ACS patients with complex coronary anatomy.62 In summary, there is a particular interest regarding antithrombotic therapy type and duration in this subset of patients, with decision-making mostly based on clinical presentation, baseline bleeding, and ischemic risk, as well as the performed stenting strategy. Factors affecting antithrombotic treatment in patients undergoing LM and/or bifurcation PCI are shown in Figure 2.29,52,63
Conclusion
In the recent era of second-generation DES, PCI is gaining ground over CABG in revascularization of LM-CAD and complex bifurcation lesions. Optimal antithrombotic treatment has yet to be defined, though; prolonged DAPT has shown some benefit regarding adverse cardiovascular events, with a caveat of potentially increased bleeding rates. Additionally, selecting a more potent P2Y12 inhibitor seems to be a reasonable choice in patients with low hemorrhagic risk, although more randomized studies and data are required to support their use in selected patients.