The prognosis of cardiogenic shock (CS) patients admitted to the intensive care unit (ICU) depends not only on that of the underlying cardiac disease and severity of shock, but also the involvement of other organ systems, the patient’s age and comorbidities.1,2 The mainstay of management is to maintain organ perfusion and a favorable homeostatic and metabolic milieu while identifying and treating reversible pathological processes until the patient recovers with or without acute mechanical circulatory support (MCS).3 Optimal management requires a detailed, systematic assessment of all organ systems, balancing the risks and benefits of any investigation or intervention on the patient, while avoiding the complications associated with critical illness and ICU admission.
General Principles
The need for ICU admission is usually obvious, such as in the case of post-operative CS requiring multi-organ support or after cardiac arrest; however, the indication for admission is not synonymous with a diagnosis of CS, which relates to the underlying pathophysiological status of the patient, as well as any acute precipitant. For every admission for CS, a precise description of each component of the patient’s cardiac pathophysiology is needed to help plan interventions to treat the new pathology and manage any additional, contributing cardiovascular comorbidities. Management must respect all the principles of critical care, including care bundles and multidisciplinary team (MDT) practice, while aiming to resolve CS and avoiding complications.4-6
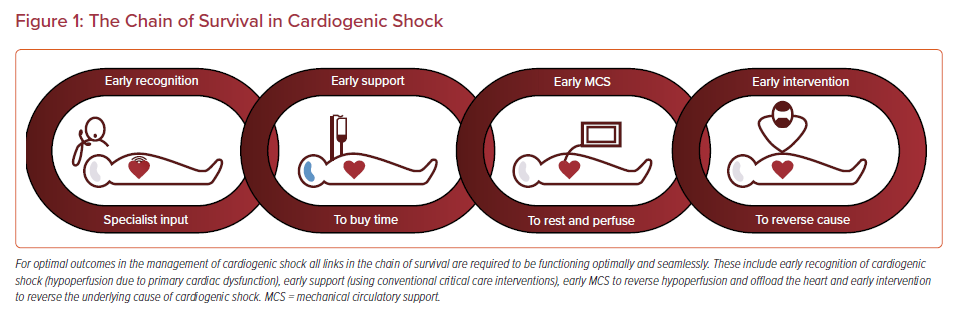
At the point of referral or when CS is recognized, an emergency shock MDT should be convened to define the treatment pathway within the chain of survival in CS (Figure 1).7 This includes rapidly determining and executing the critical care and MCS strategy and/or transferring to a CS center for ongoing management.8,9 Major decision-making in CS should be multidisciplinary; planning interventions according to shock protocols in a timely manner, taking into account what is realistically achievable and has an acceptable risk profile while respecting the principles of shared decision-making.10,11 This is particularly important when considering the lack of high-quality evidence to support many interventions used for CS and that its prognosis is worse than many other malignancies. Where the precipitating deterioration is so extreme, symptom control and palliative care may be the most appropriate course of management.12
This review is concerned with the assessment management of CS in critical care, highlighting where care would be different from critical care patients who do not have CS. It does not cover management of specific acute MCS devices.
Cardiovascular Assessment
Monitoring and integration of all available data from clinical examinations, biomarkers, and specialist investigations should be used to optimize patient management with several key features being particularly relevant to CS patients concerning static variables:
- Tachycardia may be of benefit in some patients, in particular those with restrictive right and/or left ventricular filling where cardiac output (CO) is dependent on heart rate (HR). Treating tachycardia per se in CS is not recommended.
- Although relative and/or absolute hypotension is pathological, it may be necessary, for example after aortic root surgery, in the early management of aortic dissection or, on occasion, with MCS.
- Despite cardiac index (CI) values <2.0 l/min/m2 being associated with a poor outcome after cardiac surgery, the required CI will vary depending upon the underlying pathology. An index of <3.0 l/min/m2 post-MI is associated with a significantly increased mortality. In MCS, the CO from the device and the patient need to be considered in parallel.
- Right-sided filling pressures are elevated in disease, but they may be normal for the individual patient. Where ventilated, the measured central venous pressure and waveforms must be interpreted in the context of the ventilator settings and the known right-sided pathophysiology. In the context of isolated MCS of the left heart in particular, the right heart must be taken into consideration, and features of right ventricular (RV) failure should not be ignored.
- Although peripheral edema is generally only of cosmetic concern, in the critically ill cardiac patient with impaired RV function, the presence of marked peripheral edema may signal the presence of gut mucosal edema, with an increased tendency for gastrointestinal (GI) failure, the associated risk of ileus and increased intra-abdominal pressure, and a consequent potential fall in CO.13-18
Pulmonary artery (PA) catheterization allows for the opportunity to measure global CO, oxygen consumption and delivery, however, this does not reflect regional differences. Regional resistance is affected by numerous factors, including the neurohormonal response related to inflammation and the sympathetic nervous system, and local autoregulatory factors, all of which are altered in CS.19 Thus, although global delivery may be adequate, key organs may be relatively under-perfused. These include the GI tract (where historically gastric tonometry was used and splanchnic/hepatic saturations measured), and the brain where near-infrared spectroscopy, continuous EEG post-arrest, daily trans-cranial Dopplers and repeated CT scanning is used as a neuro-MCS protocol in some centers.20 Local monitoring of perfusion and oxygen delivery, particularly of organ systems that cannot be readily supported, and/or drivers of the inflammatory response to critical illness may allow therapeutic interventions to be adjusted to improve outcomes in CS. Currently these are largely experimental tools, however, there should be a low threshold for suspecting inadequate local oxygen delivery, in particular where CO remains borderline and/or in the presence of high vasopressor requirements. Emerging technologies are increasingly being used to monitor CS patients who are receiving MCS and this may become routine in the future.21
Pacing and Cardiac Electromechanics
Although CO is a product of stroke volume (SV) and HR, autonomic control over HR occurs rapidly in response to changes in baroreceptor activity. Changes in HR may affect ventricular filling and therefore affect SV in cardiac patients. The intracardiac and autonomic reflex interactions of HR and SV are complex and non-linear. The duration of systole (in the absence of ischemia and rate-related conducting system disease) is relatively fixed, and therefore the time available for diastole is determined by the RR interval. The time required, used and/or available for ventricular filling varies significantly with pathology. This is well-recognized in valvular disease but less so in isolated left ventricular (LV) and/or RV disease and pulmonary hypertension.22 Assessment of the optimal HR for each CS patient should be undertaken as a matter of routine and optimized using echocardiography where possible.23
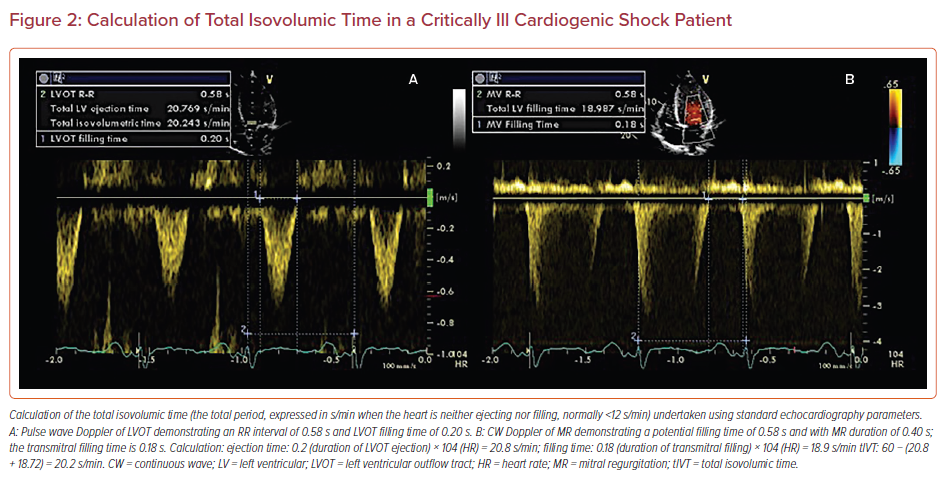
In addition to optimizing HR, as the contribution of atrial systole varies with pathology and age, the optimal atrioventricular (AV) interval will also vary between patients and at different HRs. The optimal AV delay should be the shortest possible that allows completion of diastolic ventricular filling without truncating the atrial contribution, and can be determined by systematic alteration of both AV delay and HR while recording ventricular filling, total isovolumic time and SV using echocardiography.24 Although not routinely assessed, optimization of HR and AV delay provide a way to increase CO without the use of positive inotropic agents, and in some patient populations this may lead to an increase in stroke volume of up to 43% and has the potential to reverse CS (Figure 2).25-27
Acute resynchronization using biventricular pacing is not generally recommended in CS. However, the theoretical benefits compared with the use of positive inotropic agents, including improvement of cardiac function and efficiency without increasing myocardial oxygen consumption, are clear. Small case series and case reports using acute resynchronization in CS to improve CO have been published. However, as global LV electromechanical dyssynchrony may be seen in up to 24% of critically ill cardiac patients, there is potential for use of this technique in carefully selected cases.28 Each patient with refractory CS should be considered on an individual basis, in particular those who are most likely to benefit from the intervention, such as those with LV failure and/or clear evidence of electromechanical dyssynchrony, and discussed with the MDT.29
Systemic, Pulmonary, and Filling Pressures
Blood pressure (BP) is physiologically autoregulated through baroreceptor arcs. Although it is a static variable used in many definitions of CS, the correlation of BP with CO is poor, in particular in the presence of cardiac disease where local changes in vasomotor tone maintain BP, even in the presence of hypovolemia and severe ventricular dysfunction, particularly acutely in younger patients.
In CS, as with any critically ill cardiac patient, coexisting pathology may demand relative hypotension, for example acute aortic dissection, post-aortic root surgery, and in certain MCS settings. By contrast, diastolic aortic root pressure needs to be maintained (usually >40 mmHg) for other cardiac pathologies to maintain coronary perfusion pressure. Examples include pulmonary hypertension where right coronary filling becomes limited to diastole alone or severe LV hypertrophy with elevated LV diastolic filling pressures. Additionally, comorbidities and other pathologies may determine that optimal BP for a particular patient is targeted and maintained, irrespective of the underlying cardiac pathology. Examples include maintenance of cerebral perfusion pressure after cerebrovascular accident or traumatic brain injury, or possibly renal perfusion pressure in a patient who has previously had hypertension. A significant change in BP without any changes to critical care interventions must prompt investigation into the underlying cause and this should be corrected where required.
PA pressures are relatively infrequently measured in ICU due to a combination of concerns regarding the safety of PA catheters and the ability to estimate PA pressures using transthoracic ECG.30 PA catheterization should, however, be used in cases of CS where uncertainty exists, such as with mixed shock and where there is RV dysfunction. Any fall in PA systolic pressure in response to interventions must be interpreted in the context of RV function and pulmonary vascular resistance as a failing RV will be unable to maintain high PA pressures. New, unexplained or disproportionate pulmonary hypertension should prompt investigations into the underlying cause and appropriate interventions to minimize the effect on RV afterload and CO should be put in place.
Central venous pressure (CVP) is frequently erroneously used to guide volume replacement. There is a poor correlation between CVP and volume responsiveness (positive predictive value 47%).31 Similarly the pulmonary capillary wedge pressure (PCWP) has only a 54% positive predictive value when used to determine volume responsiveness.31 In general, dynamic parameters used to predict volume responsiveness have not been validated in CS. CVP and PCWP waveforms can be useful, suggesting pathological processes that will fundamentally alter patient management.32 Demonstration of a dominant (diastolic) descent on the CVP in the presence of an elevated filling pressure may suggest restrictive RV physiology and demand an alteration of ventilatory strategy.33 Presence of pulmonary capillary V waves in the presence of an elevated PCWP may suggest or confirm the diagnosis of severe mitral regurgitation, although with a relatively low sensitivity and specificity.34
Interventions: Pharmacology, Ventilation, and Acute Mechanical Circulatory Support
Supportive interventions routinely used in ICU may need to be adjusted for CS patients as there may be subtle but important differences in their application depending upon the underlying pathology and the severity of CS. These include: the use of vasoactive drugs in specific settings; intubation and ventilation; feeding; and GI and hepatic assessment.35
Ensuring Appropriate and Optimal Cardiovascular Therapy
The hemodynamic status of any CS patient must be interpreted in the context of the pharmacological and ventilatory support they are receiving. Drug therapies must be assessed in turn in the context of the underlying pathophysiological process and adjusted accordingly, guided by appropriate monitoring.13 Although there is little evidence to support the use of any particular inotropic/vasopressor agent, some specific conditions and situations deserve particular consideration.
Pulmonary Hypertension
High dose constrictors should be avoided; however, it is crucial to maintain adequate aortic root pressure of the hypertrophied, hypertensive RV. Vasopressin at low dose can be considered as a noradrenaline-sparing agent. Although there are theoretical benefits of levosimendan above other vasoactive agents, evidence is insufficient to recommend routine use. Inhaled pulmonary vasodilator therapies may be of benefit, but caution should be used for administration of systemically active pulmonary vasodilators, as they may cause profound hemodynamic instability in CS. Their prescription in patients with adult congenital heart disease (ACHD) with potential for bidirectional shunting should only be given by clinicians with particular expertise in this field, as profound pulmonary vasodilatation may result in a significant fall in CO in certain cases.36
Left Ventricular Hypertrophy
Patients with significant LV hypertrophy with CS are at risk of developing subendocardial ischemia in the presence of inadequate aortic root pressure and dynamic LV outflow tract obstruction in the presence of under filling and/or injudicious use of β-agonists. A particularly challenging combination is when it exists in the presence of RV failure. Here the balance of inotropic agents can only be managed when guided by echocardiography, and percutaneous MCS may be required.
Known Heart Failure
When a patient is stable or improving from CS on inotropic support, standard heart failure medications may be cautiously reintroduced provided there are no contraindications. Angiotensin-converting enzyme inhibitors can be introduced concomitantly with low-dose inotrope infusion at the weaning stage. Acute β-blockade in CS is, however, potentially dangerous, and should be considered where other inotropic therapy which does not share the same pharmacological pathway, such as levosimendan, is administered. Generally, this should be undertaken in collaboration with the heart failure MDT and not acutely in the context of CS.37
Acute Coronary Syndromes
Where it is vital that anti-platelet agents are active and when there is doubt that the patient is absorbing their drugs, these should be changed to IV/per rectal preparations where possible until reliable GI function is restored. A similar approach should be taken for drugs administered subcutaneously. Where a patient is receiving MCS, anti-platelet and anticoagulation strategies should be individualized including discussion with the interventional cardiologist and hematologist where bleeding/thrombosis occurs and/or thrombocytopenia develops.38
Arrhythmia
Anti-arrhythmic drug therapy should be regularly reviewed for all CS patients. Where inappropriate bradyarrhythmias occur in the context of amiodarone therapy, consideration should be given to stopping the infusion, even where back-up pacing is available. Significant arrhythmias can be masked by venoarterial extracorporeal membrane oxygenation (VA-ECMO) and a high index of suspicion should be maintained, in particular when a weaning study is proposed. Where tachyarrhythmias are persistent or tachycardia and bradycardia coexist and limit CO, expert consultation with electrophysiology is indicated as acute ablation may be required, in particular for atrial flutter or ventricular tachycardia.39
Ventilation in Cardiogenic Shock
In low CO states where patients are allowed to breathe spontaneously, there is disproportionate redistribution of blood flow to the muscles of respiration, potentially at the expense of perfusion of other vital organ systems.40 Guidelines recommend that intubation and ventilation should be considered early in the management of acute heart failure and CS using lung-protective ventilatory strategies.3 If intubated and ventilated, the drive should be to wean the patient from the ventilator as soon as the underlying precipitating factors have been addressed, CS has resolved and the patient meets parameters for potential successful weaning.
Ventilatory Weaning Post-Cardiogenic Shock
Weaning and extubation cause significant physiological stress. Reducing sedation results in increased work of breathing, HR, BP, SVR, and PCWP, and withdrawal of the airway is associated with a catecholamine surge and a significant increase in rate pressure product.41 Further, in a patient with a borderline CO state, return to spontaneous breathing may result in deleterious blood flow redistribution. When a cardiac patient fails to wean from mechanical ventilation and all respiratory parameters have been addressed, a systematic approach to diagnosing or excluding a cardiac cause should be sought.
Alveolar/interstitial edema: Evidence for increased left atrial pressure and/or LV end-diastolic pressure should be sought, both at rest and on attempted weaning. A range of echocardiographic parameters can be used to estimate left atrial pressure, but not all are well validated in the ventilated patient or in MCS. A combination of parameters has been recommended depending on the clinical setting. Lung ultrasound may be used to demonstrate the dynamic appearance of B lines, indicating the development of interstitial edema.25
Demonstrating reversibility: Echocardiography (either targeted physiological, pharmacological, or volume/pressor loading, depending upon the potential underlying cause) is pivotal in determining what the cardiac response to weaning is and potentially suggesting an intervention that may result in successful weaning.25 Potential causes to diagnose or exclude are:
- Regional or global MI;
- Inotropy mismatch: left, right or biventricular;
- Chronotropy mismatch: inappropriate tachycardia/bradycardia/AV delay;
- Lusitropy mismatch;
- Afterload mismatch: fixed/dynamic outflow tract/cavity obstruction; and
- Preload mismatch: dynamic valvular regurgitation/limitation of filling by impaired venous return.
In some patients receiving MCS, ventilatory weaning may be appropriate while they are still on support and before the cause of CS has been completely resolved. Ideally, decisions regarding the ventilatory weaning strategy should be made when MCS is set up, and every day thereafter.
Right Heart and Ventilation
The RV fails due to an increase in afterload and/or a reduction in coronary perfusion. Both situations may be present in CS, further exacerbated by any increase in afterload due to positive pressure ventilation/pulmonary disease and/or the presence of pulmonary embolism. Restrictive RV physiology may be seen in up to 43% of critically ill cardiac patients and when present in patients with a borderline CO due to RV failure, ventilatory parameters should be altered accordingly.42 The pathognomonic presystolic A wave (which may be responsible for up to 25% of stroke volume from the right heart) may be obliterated during delivery of a positive pressure breath. Simple ventilatory measures to protect the RV in this setting involve avoidance of those factors that are associated with RV restriction in critical illness: hypercapnia, acidemia, and hypoxia, as well as minimizing positive end-expiratory pressure, mean airway pressure, and shortening the inspiratory time. Although the literature suggests positive pressure ventilation should be avoided in RV failure (in particular in certain ACHD patients), it may serve to reduce pulmonary vascular resistance – maintaining normoxia and normocarbia and reducing bibasilar atelectasis – and if used judiciously can improve CO.43 Other respiratory interventions to protect the RV include aggressive drainage of pleural effusions and pulmonary vasodilator therapy. All these considerations become particularly important when a CS patient is receiving isolated left-sided MCS with borderline RV function.
Acute Mechanical Circulatory Support
Short-term MCS should be considered in CS, aiming to reverse critical end-organ hypoperfusion and hypoxemia and buy time for interventions to reverse the underlying cause and offload and rest the myocardium, ideally allowing recovery (Figure 1).3 High-quality evidence regarding outcomes with acute MCS are scarce and it requires specialist multidisciplinary expertise for patient selection, implantation and ongoing management.3-5 Where a patient has an Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) class I or II, or The Society for Cardiovascular Angiography and Interventions (SCAI) shock classification C–E, early consideration for acute MCS is recommended.3-5,8-10 Studies have demonstrated that a standardized team-based approach in high-volume centers, using care protocols with early MCS coupled with close monitoring (invasive hemodynamics, biomarkers and echocardiography) has the potential to improve outcomes.3-10
Gastrointestinal, Hepatic, and Renal Considerations
Acute GI dysfunction occurs in approximately 10% of critically ill patients and is increasingly recognized as being an important determinant of outcome (mortality 43.7% versus 5.3%; ICU stay 10 days versus 2 days; duration of mechanical ventilation 8 days versus 1 day).44 Enteral feeding should usually be commenced within 48 hours of admission but may be delayed in patients with a SCAI CS score of D or E. When started, a low-dose/trophic rate should be considered, in particular in the presence of RV dysfunction. Relevant indications for stress ulcer prophylaxis in CS include shock itself, acute kidney infection requiring renal replacement therapy, mechanical ventilation >4 days, coagulopathy (platelets <50,000, prothrombin time 2 × upper limit of normal), anticoagulation, arterial hypotension and dual antiplatelet therapy.44
Non-occlusive mesenteric ischemia is a potentially catastrophic complication of CS, resulting from low CO and local splanchnic vasoconstriction. Manifestations vary from transient GI failure to a fulminant necrotic GI tract. The situation is further compounded in the presence of RV failure, where venous congestion results in concomitant mucosal edema and disruption of the microcirculation and intestinal barrier. GI perfusion is complex and precarious in CS. Enteral feeding increases flow in the superior mesenteric artery, which is decreased with parenteral feeding. While in shock after cardiac surgery, low to moderate vasopressin doses may induce intestinal vasoconstriction. Further, routine nursing care, such as airway suctioning, repositioning, sedation hold, may further impair splanchnic perfusion. Where splanchnic ischemia is suspected, any reversible causes should be addressed and enteral feeding should be low dose only, or potentially withheld in the short term. There is evidence to support the use of dobutamine plus noradrenaline to increase portal circulation, however this has not been supported by large-scale clinical trials.45,46
Elevation of serum bilirubin concentration >2 mg/dl within 48 hours of ICU admission occurs in 11% of critically ill patients and is an independent risk factor for poor prognosis, with a longer median ICU stay and increased hospital mortality (30.4% versus 16.4%; p <0.001). A marked increase in serum aminotransferases and lactate dehydrogenase is typical for hypoxic hepatitis which can range from isolated abnormal labs to fulminant acute liver failure.47 An international normalized ratio >2 is an independent risk factor for mortality and a rapid decline in serum aminotransferases is seen once CS is corrected. Where liver dysfunction is diagnosed, cardiovascular support should be used to improve oxygen delivery to the liver while maintaining low filling pressures.47,48 This includes:
- Aggressive reduction in RV afterload (pulmonary vasodilators, minimizing impact of ventilation, consider drainage of pleural collections).
- Consideration of reconfiguration of MCS if appropriate, such as the addition of right-sided support, upgrade to VA-ECMO, modification from veno-atrio-veno (VAV)-ECMO to veno-veno-atrio (VVA)-ECMO.
- Maintenance of adequate, but not excessive, filling pressures.
- Treatment of arrhythmias, in particular atrial flutter.
- Consideration of an increase in positive inotropic agents.
- Avoidance of factors known to reduce splanchnic flow.49
Any hepatotoxic agents should be stopped, including antimicrobials associated with liver dysfunction. N-acetyl cysteine infusion can be used, but its benefits are not proven in this patient population.
Patients with CS and high inotropic requirements ± acid-base disturbance may warrant early hemodiafiltration, however, there is no high-quality evidence to support this approach.48 Despite advancement in filter technology, initiation of continuous veno-venous hemofiltration or hemodiafiltration may cause profound hemodynamic instability in CS, related to volemic changes ± the extracorporeal circuit. Where a patient is profoundly vasoplegic, the response to pressers may be poor/unpredictable, and filtration should be initiated only once the agents to which a patient will respond have been determined and overseen by a senior practitioner. This is particularly relevant in patients with LV hypertrophy ± pulmonary hypertension ± relative hypotension where a fall of systolic BP ≥30 mmHg should be anticipated and avoided where possible. In CS patients on MCS this is generally not a concern and any instability can be readily offset with volume resuscitation.
Cardiopulmonary Resuscitation
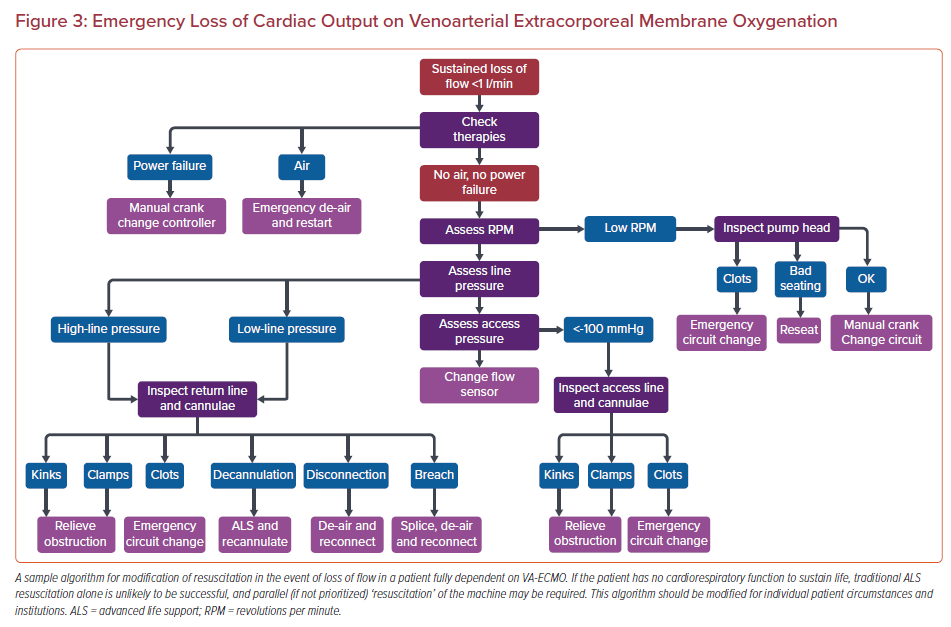
Cardiac arrest is managed in the usual manner in patients with CS and in cases of refractory arrest, extracorporeal pulmonary resuscitation may be considered according to current guidelines.50 When already receiving acute MCS, the situation is more complex. In a patient with insufficient cardiorespiratory function to support life (full VA-ECMO support) the priority in the event of emergency loss of CO (eLOCO) from circuit failure is directed towards resuscitation of the circuit. By contrast, where the circuit fails for a patient on univentricular support who is close to weaning, resuscitation should be directed initially towards the patient while simultaneously troubleshooting the circuit.51 The resuscitation strategy cannot, however, be defined solely by the type of support as it also depends upon the degree of support required. Each critical care safety briefing/handover must therefore include the resuscitation strategies and priorities in the event of either cardiac arrest and/or eLOCO to minimize any interruption to circulation and oxygenation (Figure 3).
Organ Donation and Withdrawal of Life-sustaining Therapies
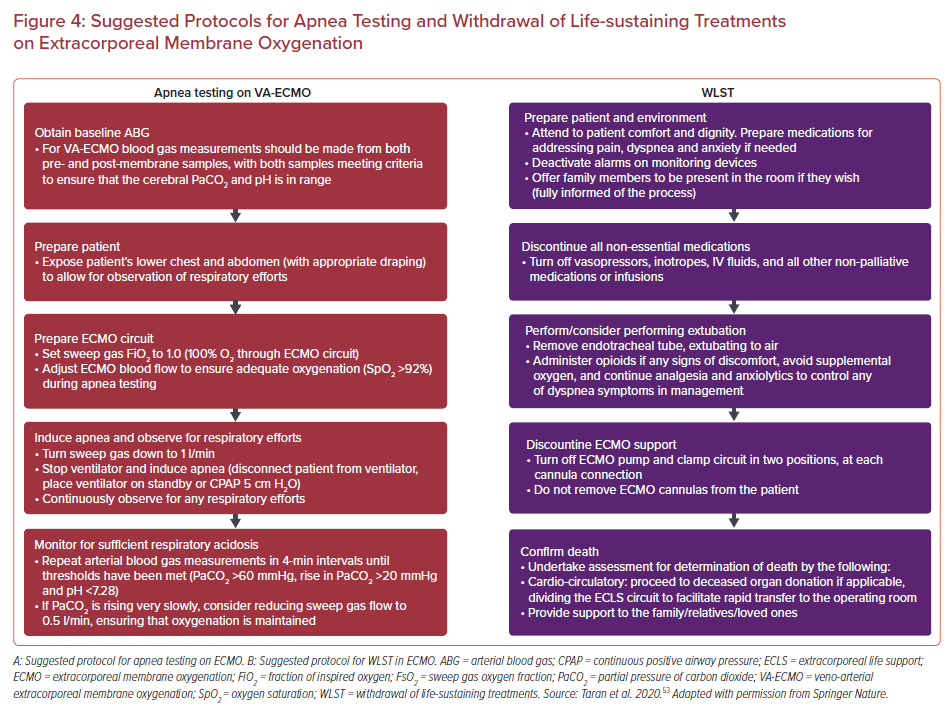
According to data from the Extracorporeal Life Support Organization, 55–79% of VA-ECMO patients could be potential organ donors.52 Although the principles underpinning end of life care and decisions for organ donation are no different in this patient population, the apnea component of determination of brain death on VA-ECMO is a particular challenge, requiring criteria to be fulfilled for testing to be valid, and withdrawal of life-sustaining-therapy can prove challenging for the medical team and the patient’s relatives. Specific guidance has been issued to support clinicians in this process (Figure 4).53
Conclusion
Although many of the principles of critical care apply to patients with CS (with and without acute MCS), there are some important differences that should be respected to optimize patient care. Further, there are certain situations where the presence of CS/MCS will significantly influence critical care decision-making and management. This most complex, challenging and rewarding component of cardiac critical care is likely to expand significantly in the next few years as we gather more data. In the interim, integration of holistic, high-end critical care with the principles of cardiac physiology and, where applicable, extracorporeal support based on the best available evidence is mandated to provide CS patients with the best chance of an optimal outcome.