Several advances in the field of echocardiography have led to improvements in the accuracy of stress echocardiography (SE) and further strengthened its established diagnostic and prognostic role in patients with known or suspected coronary artery disease (CAD). 1,2 In addition to its role in CAD, SE can be used to assess the severity of valvular heart disease and for detection of occult pulmonary hypertension. Stress-provoked ischemia can be induced by increasing myocardial oxygen demand by exercise, by administration of pharmacological agents, or by transesophageal atrial pacing. 3
Continuous advances in ultrasound imaging technology have maintained a leading role for SE in cardiovascular disease diagnosis. These advances include: the introduction of synchronized display of rest and stress images side by side using digital acquisition; improvement of endocardial detection by tissue harmonic imaging;4 contrast agent use to achieve left ventricular opacification (LVo), enhancement of endocardial border definition (EBD), and detection of myocardial perfusion;5 3D imaging; and myocardial tissue quantitative techniques (tissue Doppler, myocardial strain, and strain rate imaging). These technologies further improve the accuracy and reliability of SE.
Methodology and Interpretation
Choosing the type of stress test depends on the patient’s ability to exercise, presence or absence of baseline electrocardiographic (ECg) abnormalities, and whether or not it is important to assess myocardial viability. In patients who are able to exercise, the protocol includes a treadmill exercise test or a stationary bicycle exercise (either upright or supine). Exercise increases flow on the basis of an endotheliumdependent, flow-mediated coronary dilation of the distal coronary resistance vessels to supply myocardial oxygen requirements. Pharmacological stress testing is used in patients who cannot exercise sufficiently to achieve a minimum threshold of five metabolic equivalent of task (METs). Pharmacological agents used include inotropic agents such as dobutamine and vasodilator agents such as dipyridamole or adenosine. 6 Dobutamine increases heart rate, which results in increased myocardial oxygen demand, 7 while vasodilator agents act to directly augment coronary flow. 8 Pacing SE using an esophageal probe (inducing tachycardia) is an alternative to pharmacological stress. During atrial pacing, myocardial oxygen demand is increased because of the augmented inotropic state related to tachycardia. 9
SE is almost universally performed using transthoracic imaging, usually with harmonic imaging. Transesophageal imaging was occasionally used in the setting of poor image quality; fortunately, the advent of ultrasound contrast agents has obviated this practice. Image acquisition during SE varies with the stress protocol used. usually, treadmill images are obtained within one minute of peak exercise. In bicycle exercise, a continuous monitoring of wall motion is permitted. 10,11 Digitized dobutamine SE images are viewed on a ‘quad screen’ displaying the rest, low dose, pre-peak dose, and peak dose for each of four standardized views (four-chamber, two-chamber, long-axis, and short-axis). In atrial pacing protocols, three two-minute stages (baseline, 70% of age-predicted maximum heart rate [APMhR], and 85% APMhR) are usually acquired. 9
To date, clinical evaluation of SE imaging is mainly performed using visual (qualitative) assessment based on comparison of rest and stress images for global and regional dysfunction. The diagnostic criteria of stress echocardiographic testing are centered on describing four response patterns, including ‘normal,’ ‘inducible ischemia,’ ‘fixed scar (necrosis),’ and ‘stunned’ or ‘biphasic response,’ as may be seen in dobutamine SE viability studies. Furthermore, the site, extent, and severity of abnormal myocardial function, and ischemic threshold, can be identified. 3 The subjective interpretation of SE is highly dependent on the skill and experience of the reviewing physician. In addition, it is affected considerably by image quality, which in the ‘pre-contrast era’ was reported to be the case in up to 30% of SE exams. 12 Clear definition of endocardial border is crucial for optimal interpretation. The use of ultrasound contrast agents for LVo and assessment of myocardial perfusion have added yet another dimension to SE. 5
Diagnostic Accuracy and Prognostic Role
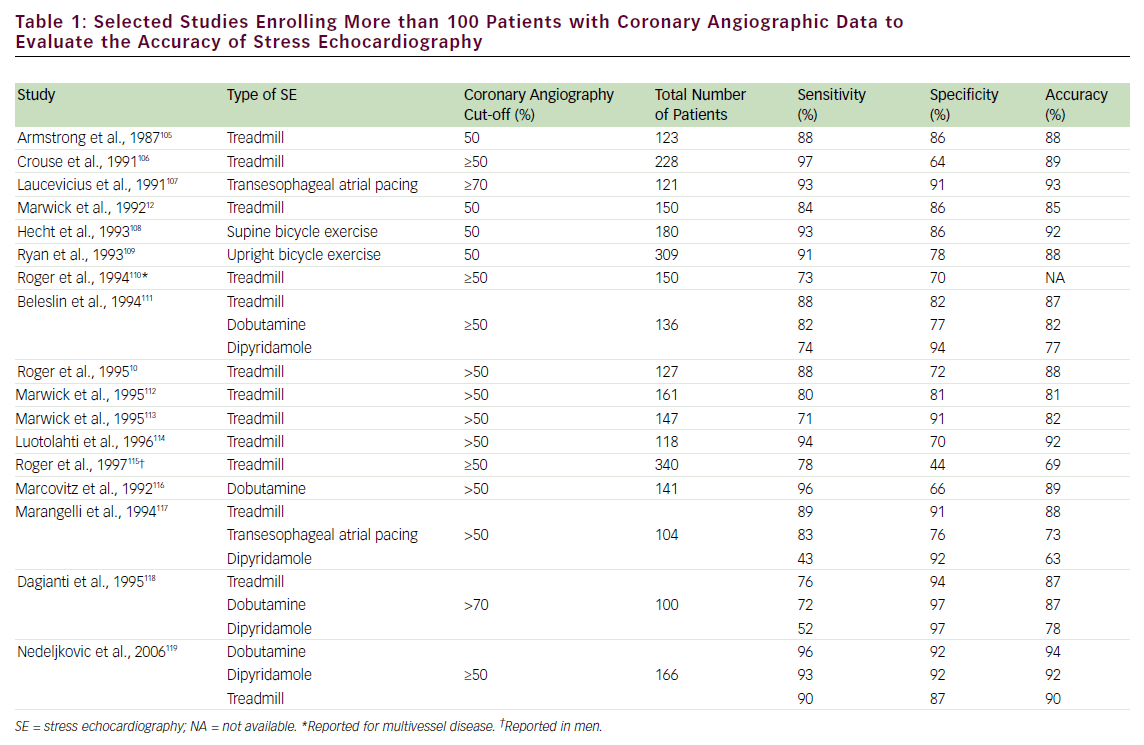
The diagnostic accuracy of SE for detecting obstructive CAD is affected by several factors, including type of stressor, adequacy of stress, severity and location of coronary stenosis, lesion morphology, number of involved coronary vessels, presence of anti-ischemic drugs, and state of collateral circulation. The choice of one stress test modality over another will depend on the individual patient characteristics. Exercise, transesophageal atrial pacing, and pharmacological (inotropic or vasodilator) SE have comparable diagnostic accuracy, as shown in selected large studies (with >100 patients enrolled) (see Table 1). The accuracy of SE generally parallels the severity of CAD; multivessel disease detection has higher accuracy than single-vessel.
The accuracy of SE has been compared with that of other noninvasive diagnostic imaging techniques such as nuclear perfusion imaging. Compared with nuclear perfusion, all types of SE—including transesophageal pacing—had marginally superior specificity for detection of CAD, while nuclear perfusion had marginally better sensitivity. 13–24 The finding that stress nuclear perfusion is slightly more sensitive can be explained by the ischemic cascade, whereby perfusion abnormalities precede wall-motion abnormalities. Interestingly, when direct comparison of contrast SE was made with nuclear imaging, contrast SE was more sensitive and slightly less specific. 25 Nevertheless, SE has numerous advantages overall: it is easier to perform, has a relatively lower cost and greater spatial resolution, and does not entail the use of ionizing radiation.
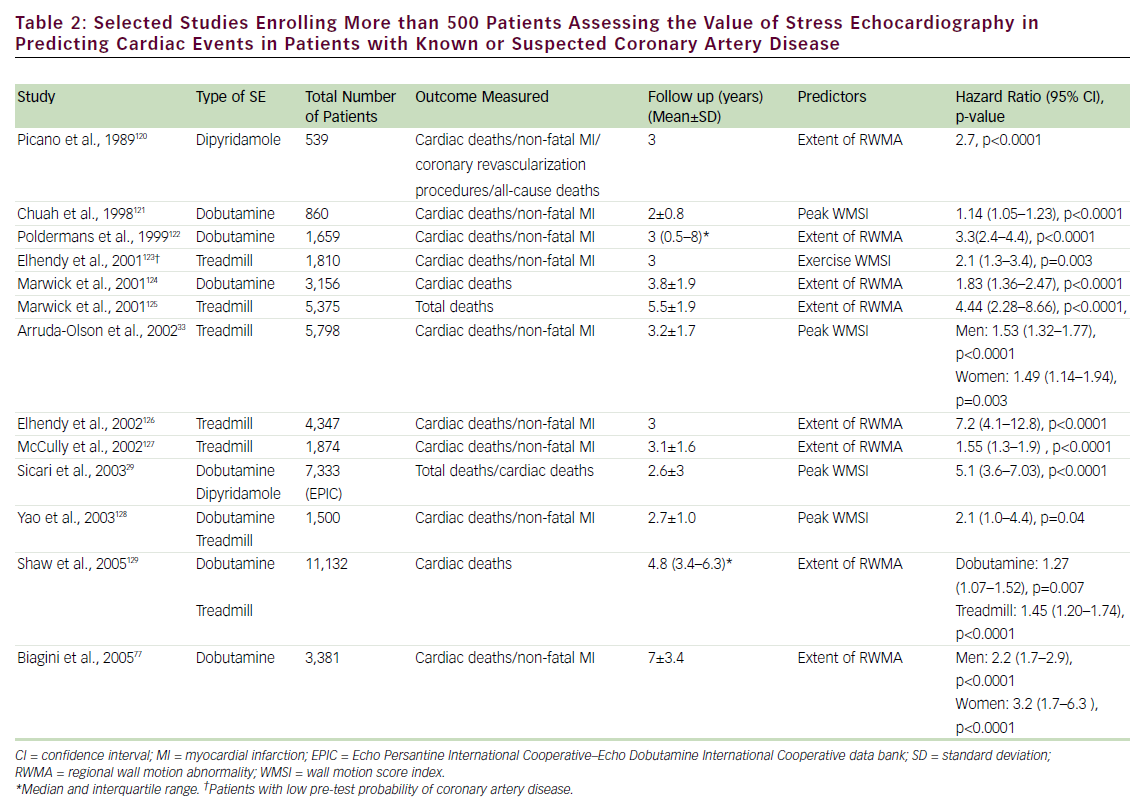
SE has been shown to play an important role in the prediction of mortality and composite cardiac events in patients with known or suspected CAD (see Table 2). A normal exercise SE is associated with a mortality of <1%/year, while normal pharmacological stress has been associated with mortality <1.5% (patients undergoing pharmacological stress have more comorbidities and, hence, a greater cardiovascular disease burden). 26–30 The prognostic value of SE has been evaluated in head-to-head comparative studies with nuclear perfusion imaging. These studies have shown a comparable event rate for normal SE and nuclear perfusion scans. 27,30–32 In addition, the prognostic value of SE has been established in specific groups of patients, including those referred for pre-operative assessment, 31 women, 33 the elderly, 34 diabetics, 35 post-acute myocardial infarction (MI), 36 post-coronary revascularization, 37 hypertensives, 38 patients with electronic pacemakers, 39 patients with left bundle branch block, 40 and patients with atrial fibrillation. 41 Although less frequently utilized, transesophageal atrial pacing SE (TAPSE) has also been found to have prognostic value as an independent predictor of cardiac events; patients with positive TAPSE are 12 times more likely to have cardiac events (deaths and MI). 42,43
SE also has a unique role in the hemodynamic assessment of valvular heart disease, diastolic dysfunction, and pulmonary hypertension, and can be used in combination with oxygen consumption testing for the differentiation of cardiopulmonary syndromes in dyspneic patients.
Cost-effectiveness
Cost-effectiveness evaluation can assist healthcare decision-makers in resource allocation for maximization of the net public health benefit; downstream costs arising from the use of a less accurate initial test may have the unintended result of increasing total costs. 44 In patients with a high likelihood of CAD, direct coronary angiography was the most cost-effective strategy despite its higher immediate cost. 45 It is in patients with intermediate risk for CAD that exercise SE can enhance cost-effectiveness. In a major study evaluating 7,565 patients undergoing both exercise testing and exercise SE, exercise SE was associated with a greater incremental life expectation and a lower cost of additional diagnostic procedures compared with exercise ECg alone. 46 In a similar study, SE resulted in a higher rate of hospital discharge compared with exercise ECg, with associated cost savings. 47
In general, the choice of SE over nuclear perfusion will depend on the overall perceived biological risk related to the use of radiation, as well as factors associated with local expertise. one of the first randomized clinical trials looking at the cost-effectiveness of diagnosis and management of patients presenting with possible CAD using several diagnostic modalities showed a satisfactory safety rate for both tests. 48 Another study addressing the cost-effectiveness of exercise SE versus stress single positron emission computed tomograpy (SPECT) imaging supported the use of SE in patients at low risk with suspected CAD and SPECT in those at higher risk. 49 Recent publications addressing concerns regarding radiation exposure from medical testing have stimulated awareness and debate in the scientific and lay communities regarding the significant contribution of cardiovascular medical testing, and nuclear cardiology in particular, to patient radiation dose. 50
Safety
Safety is a major concern for clinical, economic, and medicolegal reasons. Complications invoke excess cost and risk, and the frequency of complications affects the cost–benefit ratio. 51 A large study pooling results from 300 centers (from 85,997 patient examinations) reported an adverse event rate for exercise, dobutamine, and dipyridamole SE of one in 6,574, one in 557, and one in 1,294, respectively. The study concluded that SE is a safe method, with exercise SE being safer than pharmacological stress, and dipyridamole safer than dobutamine, possibly because of pre-selection criteria. 52 Another large registry (german Stress Echo Registry) with >60,000 tests reported similar rates of complications (0.6% for exercise, 3.6% for dobutamine, and 1.5% for dipyridamole). 53 Many other studies have confirmed these findings, with all reporting consistently low complication rates. 54–59
Recent Developments
New Technologies
A major limitation of SE is that its diagnostic criteria are based on the subjective (‘eye-ball’) interpretation of stress-induced changes in wall motion, using semi-quantitative scores. Therefore, several advances, including myocardial tissue Doppler, myocardial strain, strain rate imaging, speckle tracking, and 3D imaging, have been aimed at the development of more objective quantitative techniques to improve accuracy, decrease subjectivity, and increase reproducibility of SE interpretation.
Tissue Doppler echocardiography (TDE), using frequency shifts of ultrasound waves to calculate myocardial velocity, permits an objective measure of myocardial velocity as a means of accurately assessing regional left ventricle (LV) function during SE. It was shown that a TDEmeasured peak velocity of <5.5cm/second with peak stress had an average sensitivity, specificity, and accuracy of 96, 81, and 86%, respectively, for identifying abnormal segments at peak stress. 60 Strain (relative myocardial deformation) and strain rate (the speed of myocardial deformation) can be derived from tissue velocities either offline or in realtime. Both are relatively homogeneous throughout the myocardium and are less influenced by cardiac motion. Strain and strain rate imaging as an adjunct to visual wall motion analysis were reported to increase sensitivity of dobutamine SE in assessment of myocardial viability from 73 to 83% compared with visual assessment alone, but with specificity being unaffected. 61 other data have shown the value of Doppler- and speckle-tracking-based myocardial deformation to detect ischemia and to predict patient outcome during dobutamine SE. 62,63 Nevertheless, background imaging noise, respiratory artifacts, difficulties during exercise and bicycle echocardiography, and timeconsuming analysis are issues that still need to be resolved before these Doppler-based techniques can become standard practice in SE. 64
Realtime 3D (RT3D) echocardiography using matrix array transducers allows rapid acquisition of a 3D data set of the entire LV during SE, allowing the visualization of all LV segments by simply cropping the volumetric 3D data sets off-line, thus avoiding the potential risk of missing stress-induced regional wall motion abnormalities (RWMAs) and reducing operator dependence. Shorter acquisition time compared with 2D imaging is another important advantage of RT3D during SE. In addition, 3D technology provides more precise quantification of LV volumes and LV ejection fraction (LVEF). 65–67 one limitation of RT3D SE testing is the low temporal resolution (frame rate <20hz) and the lack of side-by-side display of 3D baseline and stress images. In addition, the multiple sub-volume data sets are combined to generate a full volume, which are subject to cardiac and respiratory motion, arrhythmias, and stitch artifacts. Currently, no sufficient evidence supports the routine use of RT3D to replace conventional 2D imaging during SE. RT3D is currently used to complement 2D evaluation in patients in whom wall motion changes are sub-optimally detected. Recently, single-beat fullvolume acquisition has become available to address these limitations, but no validation studies exist at this time for SE. Future improvements to RT3D will include the development of transducers with a smaller footprint and higher spatial and temporal resolution. In the future, use of parametric imaging with contraction front maps with 3D imaging may provide an entirely new approach in assessment of ischemia.
Appropriateness Criteria
The American College of Cardiology Foundation (ACCF) initiated an effort to develop appropriateness criteria for imaging studies in early 2005. one of the first studies to attempt to apply the recently published ACCF/American Society of Nuclear Cardiology (ASNC) appropriateness criteria for SPECT perfusion imaging to current clinical practice including SE in a single academic medical center suggested that that these criteria will apply for approximately 90% of current stress imaging, with 11% of patients remaining unclassified. 68 Recently, appropriateness criteria specific to SE have been published; 69 they include an assessment of risks and benefits of SE for several indications utilizing a score of one to nine (based on methodology developed by the ACCF to assess imaging appropriateness). In general, the use of SE for risk assessment in patients with CAD was viewed favorably, while routine repeat testing and general screening in certain clinical scenarios were viewed less favorably. The impact of the results of the appropriateness criteria on decision-making, performance, reimbursement policy, and future research is yet to be explored.
Contrast Stress Echocardiography
Reduced image quality with poor reproduction of the EBD is usually further exacerbated during stress because of chest wall movement during hyperventilation and cardiac translational movement during tachycardia. Furthermore, sub-optimal studies increase the need for additional testing. 12,70 Contrast microbubbles contain a small amount of a high-molecular-weight gas (most commonly a perfluorocarbon) encapsulated by an insoluble shell, with a rheology similar to red blood cells, and are able to traverse the pulmonary vasculature. These microbubbles remain in the vasculature as red blood cell tracers, and uniquely interact with ultrasound waves. Through advanced multipulse sequencing techniques, microbubbles produce excellent LVo and hence improve EBD, salvaging difficult echocardiographic exams and allowing accurate quantification of regional and global LV function. 71,72 To, date the uS Food and Drug Administration (FDA) has approved two commercially available contrast agents for LVo and EBD: Definity (Lantheus Medical Imaging, North Billerica, MA) and optison (gE healthcare Inc., Princeton, NJ). The development of these stable contrast agents, along with the unique methods of ultrasound delivery and reception, has resulted in robust and persistent contrast effect for LVo, as well as allowing for the realtime assessment of myocardial perfusion. At present, the routine use of ultrasound contrast agents for evaluation of myocardial perfusion has not been implemented, and is limited to research studies (off-label use). The 2008 American Society of Echocardiography (ASE) guidelines recommend that contrast agents be used when two or more endocardial segments cannot be adequately visualized, and in those difficult-to-image patients presenting for SE with sub-optimal image quality. 5 The optimal use of contrast during SE requires appropriate training and interaction of the physician, sonographer, and nurse members of the echocardiography laboratory. 5
Left Ventricular Opacification
Accuracy and Prognostic Role
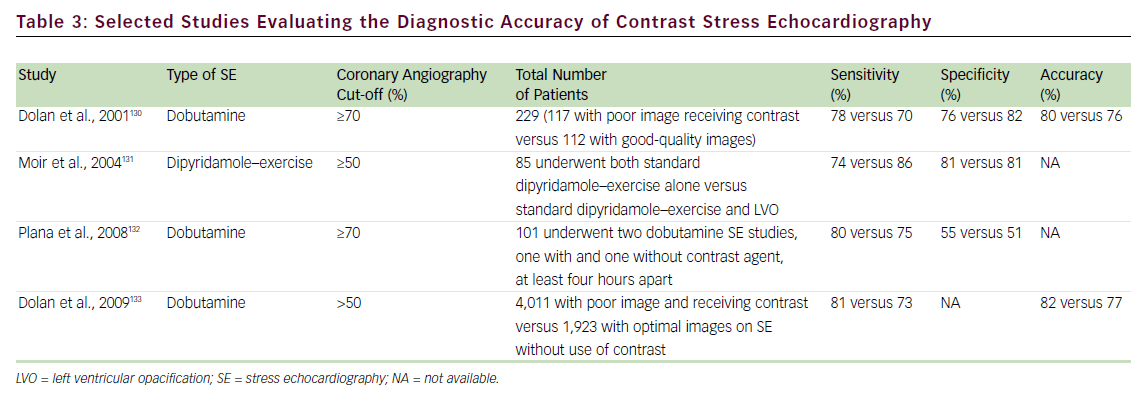
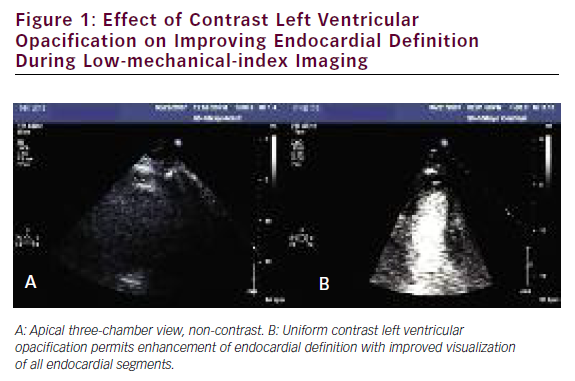
Several studies have addressed the critical clinical question of whether LVo actually improves the accuracy of stress echo for the diagnosis of CAD. Table 3 summarizes the published literature demonstrating the incremental benefit of contrast utilization for LVo in SE compared with coronary angiography. The use of contrast agents in patients with poor baseline images permitted the sensitivity, specificity, and accuracy for detecting CAD to become comparable to those for patients with goodquality non-contrast resting images. In addition, use of a contrast agent improved the reproducibility of the examination. 73,74Figure 1 shows an example of the use of contrast agents for LVo and enhancement of endocardial borders.
The prognostic role of contrast-enhanced dobutamine SE has also been evaluated. In 893 patients during a follow-up mean of 15 months, patients with positive SE (with wall motion score index [WMSI] >1.5) were 2.5 times more likely to have subsequent cardiac events (hazard ratio [hR] 2.48). 75 Another study explored the role of gender differences in the prediction of cardiac outcomes among 581 men and 309 women with known CAD undergoing contrast-enhanced dobutamine SE during a mean follow-up of 30 months. They showed that, for male patients, the independent predictor of total cardiac events was a positive dobutamine SE (hR 2.15; p<0.001), while abnormal LV end systolic volume (LVESV) response (hR 3.27; p<0.001) was predictive for female patients. Furthermore, they reported that the independent predictor of hard cardiac events for male patients was LVEF at peak stress <50% (hR 9.51; p<0.0001); however, no independent predictors were selected for hard cardiac events in females, since the hard cardiac event rate in women was very low. 76 These two studies have proved the value of using contrast-enhanced dobutamine SE in providing significant comparable information to previous studies utilizing non-contrast SE for the prediction of total cardiac events. 77,78
Cost-effectiveness
The incremental value of contrast echocardiography results from improvement in image quality, which subsequently initiates a cascade of effects: contrast permits an efficient diagnostic strategy of care by decreasing the time to diagnosis, reducing the need to use alternative diagnostic techniques, and enhancing diagnostic confidence and clinical decision-making. 73,79 These benefits were uniquely characterized and quantified in a recent study evaluating the impact of contrast echocardiography in clinical management and decision-making: additional diagnostic procedures were avoided in 33% of patients and drug management was altered in 10%. The impact of contrast use in rest echocardiography increased with worsening quality of baseline nonenhanced echocardiogram, the highest being in hospitalized intensive care unit patients where cost–benefit analysis showed significant savings—$122/patient—using contrast. 80
The benefit of contrast use appears to be especially important for patients undergoing SE. In one study it was reported that among patients with baseline sub-optimal images undergoing SE, 53% subsequently underwent an additional nuclear stress test versus 3% if they had received contrast enhancement. Thus, it is not surprising that the use of contrast in SE has been shown to be cost-effective and favorably affects the practice of performing additional tests for the same clinical indication, and results in estimated incremental savings of approximately $238/patient. 81 We also have evaluated the cost savings of using contrast agents with SE in a large single-center experience comprising 16,052 patients, of whom 3,637 (23%) received contrast. The adjusted odds ratio (oR) for having a repeat test in patients receiving contrast was 0.38 (95% confidence interval [CI] 0.29–0.47), with 16% of patients in the noncontrast group having additional testing compared with 5.8% in the contrast group (p<0.001) at 21 days. 82
Myocardial Contrast Echocardiography
Myocardial perfusion abnormalities occur as a result of limited coronary flow reserve, with a consequent decrease in myocardial contractility. ultrasound contrast agents enhance the backscattered ultrasound signal, permitting detection not only in the LV cavity, improving regional wall motion assessment, but also in the myocardium. Indeed, improved ultrasound imaging strategies for microbubble detection have resulted in routine visualization of contrast within the myocardium, and stimulated a proliferation of investigative work into the non-invasive ultrasound assessment of myocardial perfusion. Image acquisition techniques using reduced acoustic power (mechanical index 0.1–0.2), while maintaining realtime imaging frame rates (20–30hz), have allowed realtime myocardial contrast echocardiography (MCE) and simultaneous assessment of both function and perfusion.
Stress Myocardial Contrast Perfusion Echocardiography
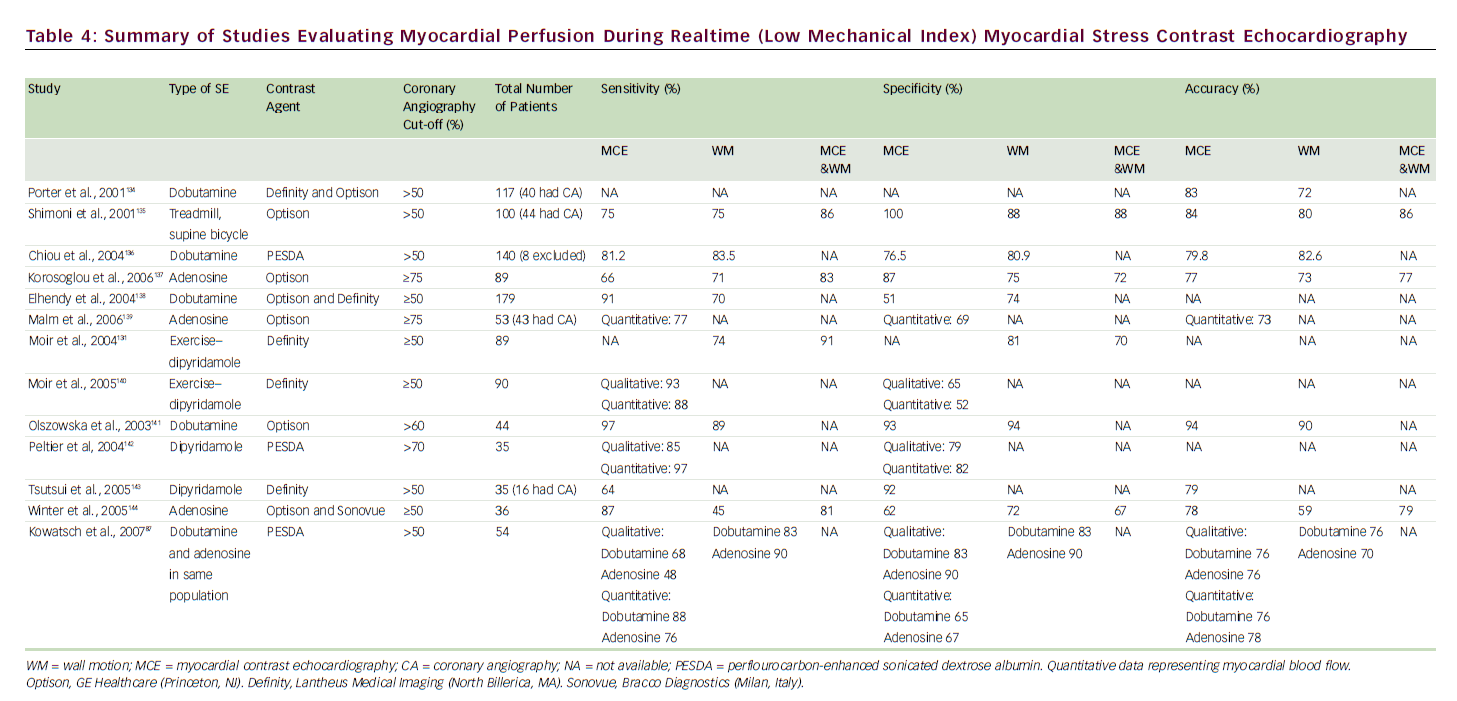
Destruction of microbubbles and observation (both qualitative and quantitative) of gradual refill (replenishment) into the myocardial microvasculature are the keys to accurately evaluating perfusion using realtime MCE. 83 A meta-analysis of eight studies comparing the sensitivity and specificity of qualitative MCE with those of SPECT/dobutamine SE for the detection of CAD showed at least equivalent (non-inferior) results. 84 A number of SE studies have employed coronary angiography as the gold standard (see Table 4) for CAD diagnosis, with reported sensitivities of 64–97% and specificities of 51–100%; MCE perfusion consistently improved sensitivity for detection of CAD over wall motion analysis alone.
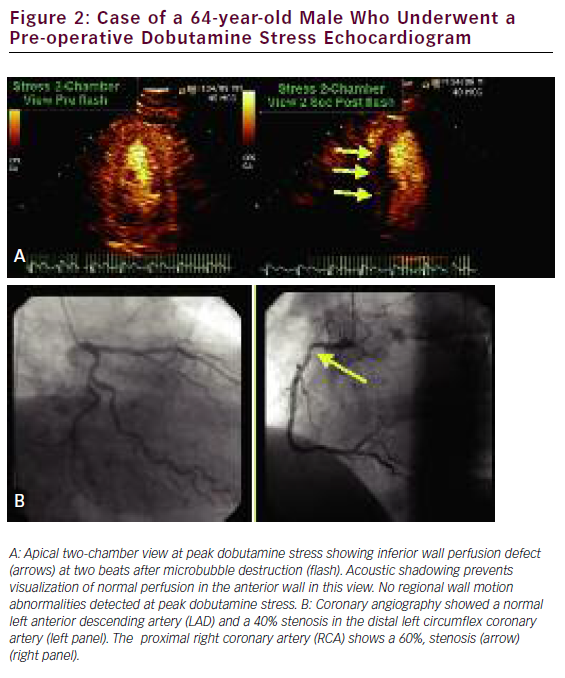
Quantification of myocardial blood flow (MBF) can be indirectly yet accurately calculated by using the rate and volume of microbubble movement through the coronary microcirculation. With continuous microbubble infusion, steady-state microbubble concentration is achieved in the blood pool. After a brief high-mechanical-index impulse to deplete the myocardium of microbubbles, replenishment is characterized by a time–intensity curve, which is subsequently fitted to the monoexponential function y=A(1–e–bt), where b represents the rate of rise in signal intensity (microbubble velocity) and A is the peak plateau of video intensity (myocardial blood volume). The product of A times b represents an index of MBF. 85 We recently performed a systematic review and meta-analysis database search of the literature to identify 627 patients who had undergone quantitative stress (vasodilator or dobutamine) MCE for detection of CAD with comparative coronary angiography and/or SPECT. We found a significantly lower weighted mean difference (95% CI) for each of the measured parameters—A, b, and Ab reserve parameters in those patients with CAD versus those without: 0.12 (0.06–0.18), 1.38 (1.28–1.52), and 1.47 (1.18–1.76), respectively (all with p<0.001). Pooled sensitivity and specificity for b reserve was 81% (76–85) and 77% (73–80), respectively, while pooled sensitivity and specificity for Ab reserve was 80% (75–84) and 81% (77–84), respectively. 86 The assessment of quantitative myocardial perfusion during SE was shown to further improve the accuracy of SE over qualitative MCE alone. 87 An example of a perfusion defect with realtime perfusion during dobutamine stress is shown in Figure 2.
Stress MCE also provides prognostic value in patients with stable CAD. Tsutsui and colleagues studied 788 patients with realtime MCE during dobutamine SE during a median follow-up of 20 months. The authors reported that patients with normal perfusion have a better outcome than patients with normal wall motion. Furthermore, abnormal myocardial perfusion had significant incremental value over clinical factors, resting ejection fraction, and WM responses in prediction of cardiac events. Indeed, abnormal myocardial perfusion was an independent predictor of cardiac events (hR 5.2, 95% CI, 3.0–9.0; p<0.001). 88 Similarly, in a recent study by Dawson and colleagues89 the incidence of non-fatal MI and cardiac death was determined in 261 patients with known or suspected CAD who underwent simultaneous dipyridamole stress MCE and 99mTc-sestamibi SPECT during a mean follow-up period of 14 months. The authors reported that abnormal results on MCE were found to be an independent predictor of an adverse outcome (oR 23, 95% CI 6–201; p<0.001) and provided incremental prognostic value over clinical variables, LV systolic function, inducible wall thickening abnormalities, and SPECT results.
3D Stress Myocardial Contrast Perfusion Echocardiography
Realtime 2D MCE is limited by the single cross-sectional 2D image, which cannot evaluate the 3D architecture of a perfusion defect. Recent improvements in 3D contrast technology include the incorporation of power modulation imaging into RT3D echocardiography, which provided an incremental benefit for visualizing intramyocardial microcirculation filled with microbubbles. 90
In 2006, Toledo and colleagues first reported on the feasibility of live 3D MCE for assessment of myocardial perfusion with transient contrast infusion. 91 They documented a two-fold increase in the measured perfusion indices during adenosine infusion. In 2007, Iwakura and colleagues reported a potential benefit of 3D MCE over 2D MCE in the assessment of perfusion and prediction of infarct size. 92 Bhan and colleagues, in 2008, reported on the feasibility of lowmechanical-index full-volume 3D MCE and showed a good agreement with 2D MCE for assessment of myocardial perfusion in 46 patients during dobutamine stress. 93 We recently demonstrated that myocardial perfusion could be assessed with 3D MCE by evaluating adenosineinduced changes in myocardial capillary blood volume and velocity. We also showed that the acquisition time for 3D MCE was shorter than that for 2D MCE. We believe that the assessment of myocardial perfusion by 3D MCE is feasible and has acceptable diagnostic accuracy compared with SPECT. 94 Currently, frame rates with 3D MCE (using power modulation) are still limited (<14hz). We anticipate that expanding technology in the next few years will further improve the utilization of contrast 3D echocardiography.
Safety of Contrast Use During Stress Echocardiography
Prior concerns about the safety of ultrasound contrast agents led to changes in the recommendations for product use in the uS. That action, in october 2007, followed post-marketing reports of four deaths within 30 minutes of contrast agent administration in patients with significant underlying progressive cardiovascular disease. An intense response from the medical community ensued, with demonstration and documentation of the safety and usefulness of contrast agents in echocardiography. The deaths, in hindsight, were thought to be ‘pseudocomplications,’ 95 and in May 2008 the FDA again revised the labeling changes to revert to the original contraindications (see below), although a box warning for use in hemodynamically unstable patients remained. The following are FDA labeling contraindications to ultrasound contrast administration:
- right-to-left, bidirectional, or transient right-to-left intracardiac shunt;
- hypersensitivity to perflutren;
- hypersensitivity to blood, blood products, or albumin (applies to optison only); and
- intra-arterial injection of ultrasound contrast agents. 96
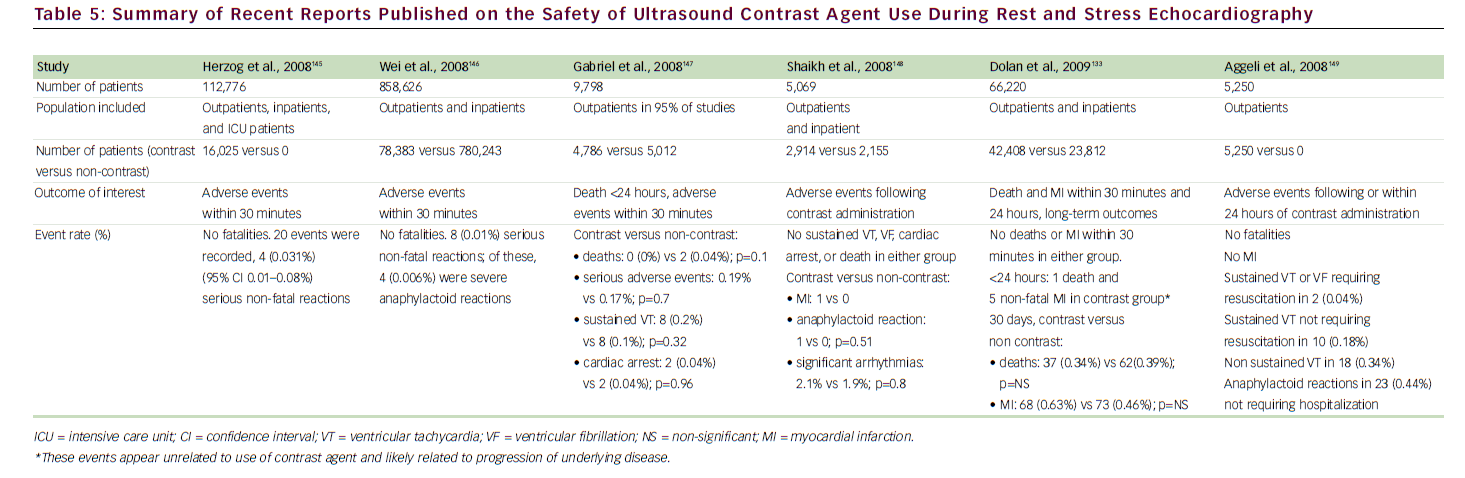
A growing body of national and international literature has continued to demonstrate the safety of ultrasound contrast agents. 55,97–100 In the medical literature, the two main concerns about the use of intravenously administered contrast agents in echocardiography are focused primarily on potential bioeffects with the use of continuous high-mechanicalindex imaging, as manifested by premature ventricular contractions or vascular/myocardial injury. 101,102 These concerns are not relevant to the low-mechanical-index and realtime MCE techniques, which are currently used for both LVo and MCE. In a recent large study of 1,486 patients reporting on low-mechanical-index imaging during dobutamine SE, compared with 1,012 patients who underwent conventional dobutamine SE without contrast agents, there was no difference between the two groups in the incidence of non-sustained ventricular tachycardia (VT), sustained VT, or supraventricular tachycardia. 99 We have also evaluated the safety of contrast use during SE in the largest cohort to date (26,744 patients) 103 and have found no evidence to suggest clinically important bioeffects. During short-term follow-up, patients who had undergone SE with contrast agents were not at increased risk for death or MI compared with those who had not received contrast agents. For long-term follow-up (4.6 years), the adjusted hRs were not different between the two cohorts for death (0.99, 95% CI 0.88–1.11) or MI (0.99, 95% CI 0.80–1.22). Contrast-agentrelated side effects were minor and infrequent. 104Table 5 shows a summary of recent studies that have addressed the safety of contrast agent use during SE.
Conclusion
SE remains a well-established non-invasive imaging modality for diagnosing CAD. SE has proved to be a reliable tool for the diagnosis of myocardial ischemia and a valid test for predicting cardiac events. Advances in imaging and display technology have resulted in enhancing and maintaining the leading role of SE as a readily available, portable, cost-effective, and non-ionizing diagnostic cardiovascular imaging modality. Newer quantitative ultrasound techniques will further improve test reproducibility and interpretation. The use of contrast significantly enhances image quality in SE, thereby increasing the feasibility of test performance, decreasing the number of non-diagnostic exams, reducing the need for alternative testing, influencing clinical management decisionmaking, and ultimately improving cost-effectiveness. Myocardial perfusion imaging in conjunction with contrast SE provides yet further incremental benefits and is emerging in its clinical application.