Hypertension remains a prevalent, major risk factor for cardiovascular disease (CVD), affecting over one-third of adults in the uS—nearly 74 million people.1 In the uS, elevated blood pressure (BP) contributes to 69% of first myocardial infarctions (MIs), 74% of cases of congestive heart failure (CHF), and 77% of first strokes.1 The total direct and indirect cost of CVD and stroke in the US for 2009 was approximately $475.3 billion. This figure includes healthcare expenditure (direct costs, which include the cost of physicians and other professionals, hospital and nursing home services, prescribed medications, home healthcare, and other medical durables) and lost productivity resulting from morbidity and mortality, otherwise known as indirect costs. By comparison, in 2008 the estimated cost of all cancer and benign neoplasms was $228 billion. CVD costs more than any other diagnostic group, and uncontrolled hypertension is a major contributor to this huge economic burden and morbidity.1
Hypertension control is at least as important as treating other major risk factors for CVD, such as dyslipidemia, glucose intolerance, smoking, and obesity, to reduce CVD-related morbidity and mortality.2–6 Various studies have shown that, for best outcomes, hypertension treatment regimens need to control BP constantly over 24 hours. Regimens that fail to control hypertension on such a continuous basis can still allow organ damage.7–11 Three main obstacles to satisfactory blood pressure control have been identified: patient-related, physician-related, and medical care system issues. The patient-related barriers include poor adherence to medication, beliefs about hypertension and its treatment, depression, health literacy, comorbidity, and patient motivation.12 The most important of these is medication adherence. It has been estimated that approximately 50% of patients with hypertension in the uS fail to keep follow-up appointments and only 60% take their medications as prescribed.13–15 Hypertension control is vital in maintaining quality of life, particularly in elderly patients.16 Furthermore, poor control of hypertension when combined with diabetes can contribute to the development of end-stage renal disease (ESRD).17–19
The renin–angiotensin–aldosterone system (RAAS) is a hormone system that maintains BP and water (fluid) balance. This system has been shown to be affected by lifestyle, diet, genetic, and other disease factors, and is a prime contributor to hypertension-related morbidity and mortality.20 As an integral part of this system, angiotensin II elevates BP, increases vasoconstriction and extracellular fluid volume, enhances sodium and water reabsorption, and enhances myocardial contractility. The angiotensin-converting enzyme (ACE) inhibitors are a RAAS-blocking class of medications and preceded the angiotensin receptor blockers (ARBs). ARBs block the activation of angiotensin receptors (angiotensin II, type 1 [AT1]), producing vascular dilation and decreasing blood pressure. The earlier ACE inhibitors may not completely block the production of angiotensin II, since there are multiple non-ACE-related means of producing angiotensin II. ARBs selectively inhibit the binding of angiotensin II at the AT1 receptor, directly limiting the effects of the increased RAAS activity that is a common feature of hypertension.21–23
This review will describe the role of irbesartan, alone or in combination with other agents, in the treatment of patients with mild to moderate hypertension and in patients with diabetic nephropathy. Similar to other ARBs, irbesartan is well tolerated and there is now a substantial body of data confirming its safety and efficacy in a wide range of patients, including those with systolic hypertension, obesity, and diabetic nephropathy, the elderly, and members of various racial/ethnic minorities who are often considered to be less responsive to RAAS blockade, specifically ACE inhibitor and ARB monotherapy.24–31
Angiotensin Receptor Blockers
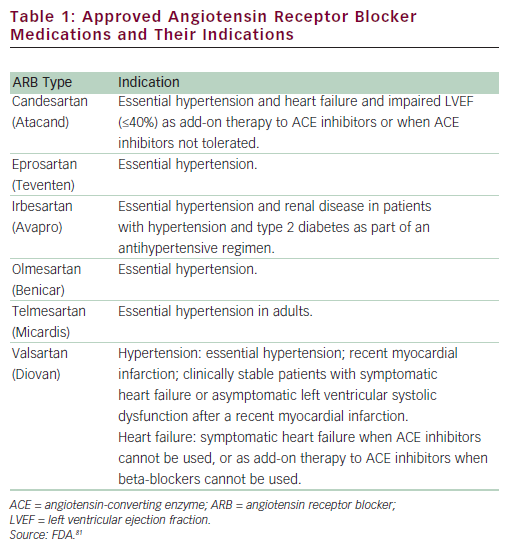
ARBs are among the newer antihypertensives and have been available for the treatment of hypertension for over 10 years. ARBs primarily lower BP by the selective blockade of the AT1 receptor, indirectly leading to arterial vasodilatation. Blockade of the AT1 receptor by ARBs reduces sympathetic nervous system activity, potentially lowers aldosterone, and improves endothelial dysfunction, without compensatory tachycardia.32–34 At present, there are seven ARBs approved by the uS Food and Drug Administration (FDA) (see Table 1) (data available from Drugs@FDA35). All ARBs are primarily indicated for BP reduction in patients with hypertension, although individual members of the class may have additional approved indications for cardiac and renal conditions, including HF, diabetic nephropathy, stroke, and systolic dysfunction following MI.
Generally, the antihypertensive potency of ARBs is similar to that of ACE inhibitors, and ARBs are useful alternatives to ACE inhibitors—and even compelling in certain situations, for example in those with diabetic nephropathy. Drugs of this class are advantageous in that they are well tolerated in most patients and adverse event (AE) profiles are not markedly different from those of placebo. ARBs are not associated with coughing, a symptom that is frequently reported with ACE inhibitors, and they have only rarely been associated with angioedema.36–40
The JNC 7 Report and Re-classification of High Blood Pressure
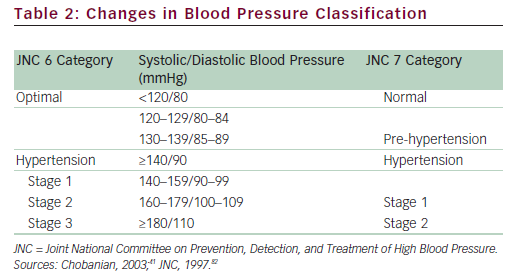
The relationship between BP and risk for AEs is continuous, consistent, and independent of other risk factors, increasing the probability of MI, HF, stroke, and kidney diseases.41 Although this review describes the use of irbesartan in patients with mild and moderate hypertension, these terms are arbitrary and increasingly avoided by various guidelines to describe the associated risk with elevated BP. There is a significant lifetime risk for CV and renal disease due to hypertension, and a dramatic increase in the risk for CV complications is noted with BPs above the normal level of <120/80mmHg. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7)41 introduced the classification of ‘pre-hypertension’ for those with BPs ranging from 120 to 139mmHg systolic (SBP) and/or from 80 to 89mmHg diastolic (DBP), identifying those individuals in whom early intervention by adoption of a healthy lifestyle could reduce BP, decrease the rate of progression of BP to hypertensive levels with age, or prevent hypertension entirely (see Table 2).41
Thus, ‘mild hypertension’ could include patients with high BP in the stratum before the arbitrary value of 140/90mmHg—specifically, those with renal disease and diabetes with a goal of <130/80mmHg. Moreover, individuals with pre-hypertension who have diabetes or kidney disease should be considered candidates for drug therapy if lifestyle modification fails to reduce their BP to ≤130/80mmHg. unlike prior guidelines, JNC 7 combined stage 2 and stage 3 hypertension into a single stage 2 category because the management approach to the former two groups is similar. Hence, both ‘moderate’ and ‘severe’ hypertension can be found in stage 2, according to the JNC 7 criteria.41 Elevated BP must be classified accurately, based on the average of two or more properly measured, seated BP readings, on each of two or more office visits. Although the use of the terms mild or moderate hypertension may identify patients based on BP elevation, risk is perhaps more accurately assessed by associated comorbidity. For the purposes of this review, the JNC 7 definition of hypertension will be used. This definition recommends treatment for all people with stage 1 and 2 hypertension.41,42
Irbesartan—Indications and Studies Showing Efficacy, Safety, and Tolerability
Irbesartan was the third ARB approved by the FDA for the treatment of hypertension and is indicated for lowering BP alone or in combination with other antihypertensive agents. In various studies this drug, when used as monotherapy or in combination with other treatments such as hydrochlorothiazide (HCTZ), aliskrinen, and ACE inhibitors,26,27,43,44 showed marked efficacy in terms of reductions in SBP and DBP during treatment periods of three months to one year and achievement of treatment goals in varied hypertensive populations. These included patients with diabetes, renal disease, obesity, and various CVDs.25,30,31,43 As with all renin–angiotensin-system-blocking agents, irbesartan should be avoided in pregnancy or in women who are breastfeeding to avoid potentially harmful effects on development in newborns.45
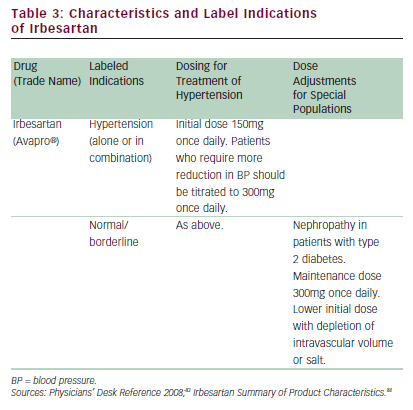
In one study on a varied population of 844 patients with hypertension receiving irbesartan and HCTZ, the reductions in both SBP and DBP compared with baseline were significant (p=<0.001), and SBP, DBP, and SBP/DBP treatment goals were met in 73, 96, and 72% of patients, respectively.27 In a randomized controlled study, treatment with irbesartan produced significant improvements in physical capability (6-minute hall-walk distance; p<0.01), exercise time (p=0.01), New York Heart Association (NYHA) class (p<0.005), and quality of life (QoL) score (p<0.005) compared with placebo.43 In these studies, irbesartan was also well tolerated when given either as monotherapy or in combination with these other agents. In the long-term treatment of hypertension, adherence to medication by the patient is a key factor determining successful control of BP.46 The results of a post-marketing survey including 4,769 hypertensive patients treated with irbesartan in Switzerland showed that more than 80% of patients were still on irbesartan at four months.47 In general, irbesartan is effective, well tolerated, and well accepted by patients—all important factors for achieving effective control of BP.47 More recently, irbesartan has been approved for use in first-line treatment (with HCTZ) of moderate and severe hypertension and is additionally approved for the reduction of progression of renal disease in patients with nephropathy and type 2 diabetes (see Table 3).35,30,48,49 Based on the findings in the IRbesartan MicroAlbuminuria Type 2 Diabetes Mellitus in Hypertensive Patients (IRMA 2) study,31 irbesartan is also approved in the Eu for use in earlystage renal disease.
The use of 24-hour ambulatory BP monitoring (ABPM) in clinical studies has the benefit of demonstrating the long-term effect on BP throughout daytime and night-time activities, and is increasingly utilized as a means of demonstrating the effectiveness of BP control.50,51 In a randomized, double-blind, multicenter, parallel-group study, ambulatory BP measurements were obtained from 426 subjects with mild to moderate hypertension who received either irbesartan 150mg or valsartan 80mg daily for eight weeks. The end-points were self-measured morning and evening DBP and SBP readings obtained at home over a seven-day period at baseline and at week eight and seated DBP (SeDBP) and SBP (SeSBP) measurements taken in the doctor’s office obtained at trough, at baseline, and at week eight. Although this study used less than optimal doses of either agent, irbesartan produced significantly greater reductions than valsartan in mean change from baseline in diastolic arterial BP (ABP) at trough (-6.73 versus -4.84mmHg; p=0.035), mean systolic ABP at trough (-11.62 versus -7.5mmHg; p<0.01), and mean 24-hour diastolic ABP (-6.38 versus -4.82mmHg; p=0.023) and systolic ABP (-10.24 versus -7.76mmHg; p<0.01). Both drugs were well tolerated. None of the discontinuations was due to serious AEs; those AEs considered related to the study drug were headache, fatigue, and dizziness, which occurred at equal frequencies in both treated groups. No serious AEs were considered to be related to irbesartan. Irbesartan was more effective than valsartan in reducing DBP and SBP at trough and in terms of overall 24-hour BP-lowering efficacy.50
Losartan, the first FDA-approved ARB, and irbesartan have different pharmacokinetic profiles.52,53 Although both drugs received approval for once-daily use, losartan may be less effective on this regimen than when prescribed twice daily. In several studies irbesartan has shown greater efficacy in treating hypertension than losartan and has proved to be at least as effective as other ARB agents.49,53–56 In a double-blind study, 567 patients with mild to moderate hypertension were randomized (1:1:1:1) to once-daily therapy with placebo, 100mg losartan, 150mg irbesartan, or 300mg irbesartan for eight weeks.55 The results showed that reductions in trough SeDBP between baseline and eight-week treatment were 3.0mmHg greater for patients treated with 300mg irbesartan compared with those treated with 100mg losartan. The reduction in trough SeSBP was 5.1mmHg greater for irbesartan than for losartan. These differences were significant (p<0.01 for both SeDBP and SeSBP comparisons). However, the overall antihypertensive effect of 150mg irbesartan did not differ significantly from that of 100mg losartan. AEs were mainly headache, musculoskeletal pain, dizziness, upper respiratory infection, and fatigue; the maximum 300mg dose of irbesartan was associated with the lowest incidence of AEs and discontinuations. This trial indicated that the maximally effective doses of irbesartan and losartan given once daily have antihypertensive effects that are significantly different at trough. These findings emphasise the clinical significance of the pharmacokinetic and pharmacodynamic differences between these two ARB medications.
Other data have confirmed the potency of irbesartan compared with olmesartan and valsartan. In a multicenter, randomized, double-blind, parallel study including 588 patients, the effect of irbesartan, losartan, and olmesartan on 24-hour ambulatory blood pressure was investigated. The study population consisted of adults with mild to moderate hypertension (mean age approximately 52 years, 62% male, predominantly white, mean baseline BP 157/104mmHg). The results indicated significantly greater reductions in mean 24-hour ambulatory SBP and DBP with irbesartan than with losartan or valsartan.57 In this study, olmesartan was shown to be comparable to irbesartan in terms of 24-hour BP control, and overall safety and tolerability were found to be similar in all treatment groups. Based on this and similar studies, similar to some other ARBs irbesartan can be considered an effective ARB for 24-hour BP control in patients with mild to moderate hypertension, but perhaps with a clinically significant greater and longer BP-lowering effect than losartan.54,57,58
In a recent study, Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction (I-PRESERVE), irbesartan did not demonstrate any benefits over placebo in terms of all-cause mortality and CVD hospitalizations.24 This trial included a cohort of 4,128 patients in a placebo-controlled, double-blind, multicenter, international trial in patients ≥60 years of age and a left ventricular ejection fraction (LVEF) ≥45%. Among the HF patients, 88% had hypertension, and this was the cause of heart failure in 64%. The primary end-point in this study was a composite of death from any cause or hospitalization for a protocolspecified CV cause. This composite occurred in 742 patients (36%) in the irbesartan group and in 763 patients (37%) in the placebo group. The frequency of these primary end-point events was calculated to be 100.4 per 1,000 patient-years in the irbesartan group and 105.4 events per 1,000 patient-years in the placebo group. The hazard ratio for this endpoint for irbesartan compared with placebo treatments was 0.95 (95% confidence interval [CI] 0.86–1.05; p=0.35). Among the secondary endpoints, the hospitalization rates for CV events in patients treated with irbesartan or placebo were not significantly different (70.6 and 74.3 per 1,000 patient-years, respectively; p=0.44). Various agents used to treat HF with a preserved ejection fraction have not proved beneficial in reducing CV morbidity and mortality. There is no FDA-indicated agent for treating HF with a preserved EF and there is no single therapeutic approach. Previous data from the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) study showed that in 1,514 patients with HF but LVEF >40%, hospitalizations for CHF were significantly lower among patients treated with candesartan compared with placebo (230 and 279 hospitalizations, respectively; p=0.017). However, candesartan is not approved by the FDA for this condition (it is approved for HF with LVEF <40%).59 The reason for there being a significant difference in hospitalization rates between treated and untreated patients in one of these studies but not in the other is likely a result of differences in the protocol-specified LVEF in the inclusion criteria (≥45% and >40%).
Angiotensin Receptor Blockers and Racial and Ethnic Minorities
Data confirming the response of various racial/ethnic groups specifically to ARBs are limited. Most clinical efficacy studies of ARBs have been underpowered with respect to African-Americans and other minorities.60 Such groups have been insufficiently represented in many trials evaluating treatments for HF. Responses to hypertension therapy vary considerably between African-American, Asian, and Caucasian populations as a result of physiological differences, and for this reason it is important that these groups are included in drug development programs. Data from some previous HF trials show that African- Americans derive less benefit than white patients from ACE inhibitors, ARBs, and some beta-blockers.
The uS is becoming increasingly racially heterogeneous as the proportion of individuals defined as minorities increases. Some minority populations show above average incidence of hypertension and CVD, including kidney disease, HF, and coronary heart disease mortality.61 Genetic differences contribute to the increased incidence of these diseases, but the primary factor influencing greater hypertension morbidity/mortality in any given subpopulation is the impact of the patient’s environment, specifically high sodium intake, high-caloric diet, obesity, sedentary lifestyle, and diabetes.61
For all populations, regardless of race or ethnicity, in the JNC 7 Report thiazide diuretics are listed as the initial therapy antihypertensive agent, especially in patients with uncomplicated hypertension. Calcium channel blockers (CCBs) are also efficacious as initial therapeutic agents. Both thiazide-type diuretics and CCBs can be effectively combined with ACE inhibitors and ARBs, which are often considered less effective as monotherapy in patients of African ethnic origin.62–65
Worldwide, Asians have high rates of diabetic nephropathy and ESRD, with a significant number of deaths among Asians with diabetes.66 Moreover, Asians may benefit from ARBs due to decreased tolerance to ACE inhibitors, primarily due to an ACE-inhibitor-associated cough.67 A retrospective cohort study conducted at outpatient clinics affiliated with an urban tertiary care hospital in Boston, uS, including 2,225 consecutive patients treated with ACE inhibitors, was conducted to identify (as the primary end-point) potential factors leading to discontinuation of ACE inhibitor treatments due to AEs. The data showed that in 19% of the total cohort, ACE inhibitors were discontinued for this reason. Independent risk factors associated with this were age, female gender, ethnicity (other than African-American or Hispanic/Latino), no previous ACE inhibitor usage, history of cough with ACE inhibitors, hypertension, and anxiety or depression. Various factors contributed to intolerance, but East Asians were more likely to develop a cough (hazard ratio [HR] 2.5, 95% CI 1.1–5.7) and hyperkalemia (HR 80.3, 95% CI 5.4–1,190) and African-Americans to develop angiodema (HR 3.5, 95% CI 1.3–8.9).68 The incidence of AEs could therefore make ARBs more appropriate therapy for hypertension in Asian populations. Hispanics/Latinos with high rates of diabetes and associated nephropathy may also benefit from ARBs.69,70
Irbesartan in Combination Therapy
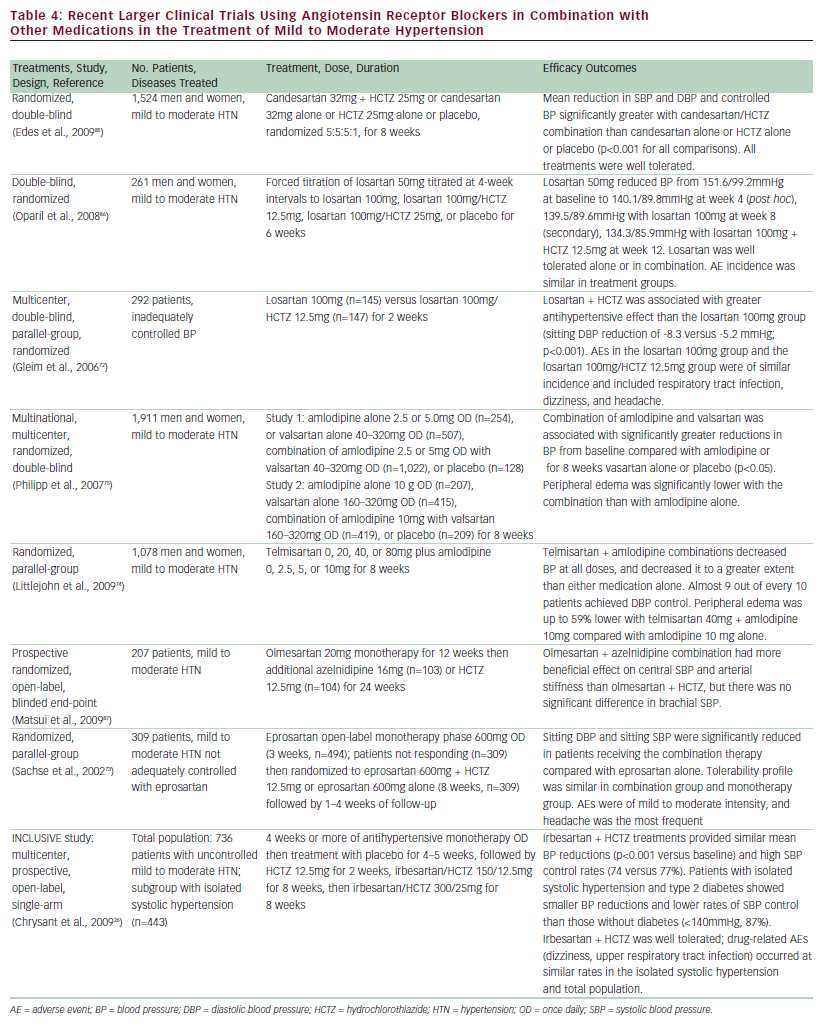
Multiple large clinical trials confirm the need for combination therapy in the overwhelming majority of patients with hypertension, as shown in Table 4. According to the JNC 7 Report, thiazide-type diuretics remain the preferred initial therapeutic agents of choice because of their excellent tolerability, relatively low cost, and multiple clinical trials demonstrating CV benefits.41 When combined with a thiazide diuretic, irbesartan safely and effectively lowers BP, including in patients not conventionally responsive to monotherapy with ARBs, such as older patients and African-American patients.
The largest trial demonstrating the benefit of irbesartan in combination with HCTZ is IrbesartaN/HCTZ bLood pressure reductionS in diVErse patient populations (INCLuSIVE).26,48 This was an 18-week multicenter, prospective, open-label, single-arm study that evaluated the efficacy and safety of irbesartan. The study included adult patients with hypertension and uncontrolled SBP on antihypertensive monotherapy ranging from 140 to 159mmHg without or from 130 159mmHg with type 2 diabetes. The primary end-point was mean change in SBP from the baseline. Treatment started with placebo for four to five weeks, then HCTZ 12.5mg for two weeks, followed by irbesartan/HCTZ 150mg/12.5mg for eight weeks, and then irbesartan/HCTZ 300/25 mg for eight weeks. In an intent-to-treat analysis (n=184), from baseline to week 18 the mean change in SBP was -23.0±13.3mmHg (p<0.001) and the mean change in DBP was -10.9±7.7mmHg (p<0.001). Mean SBP/DBP at the end of the study was 134.0±14.7/75.1±8.4mmHg, and SBP, DBP, and the SBP/DBP goal was achieved in 73, 96, and 72% of patients, respectively.27 In the total enrolled population who completed the initial placebo treatment (n=844), there were 515 Caucasians, 191 African-Americans, 119 Hispanics/Latinos, 14 Asians, and seven of other racial groups (two patients self-identified into more than one racial group).71 Mean changes in SBP from baseline (placebo treatment end) to week 18 were -21.5±13.8mmHg for Caucasians, -20.7±16.5mmHg for African-Americans, and -22.9±13.2mmHg for Hispanics/Latinos (p<0.001 for each). Mean DBP changes were statistically significant (p<0.001) and similar among racial/ethnic subgroups. In this study, drug-related AEs—dizziness in ≤3% and upper respiratory tract infection in ≤2%—occurred at a similar frequency in the isolated systolic hypertension and total population. Overall, the combination of irbesartan/HCTZ treatment achieved SPB goals in more than 75% of patients in a diverse cohort whose BP had been previously uncontrolled on antihypertensive monotherapy.26,27
In a post hoc analysis of the INCLuSIVE trial, the efficacy and safety of fixed-dose irbesartan/HCTZ in patients with isolated systolic hypertension (n=443) was compared with the total study population (n=736).26 The irbesartan/HCTZ treatment for 16 weeks provided good mean BP reductions from baseline (21.4/10.1mmHg) and high SBP control rates (74%). Patients with isolated systolic hypertension and concomitant type 2 diabetes experienced smaller BP reductions (17.9/8.7 mmHg) and lower rates of SBP control (47% achieving SBP <130mmHg) than those without diabetes (87% achieving SBP <140mmHg). Interestingly, BP reductions from baseline and SBP control rates were similar across isolated systolic hypertension subgroups (≥65 versus <65 years of age, sex, race, and metabolic syndrome status). Irbesartan/HCTZ was well tolerated, with drug-related AEs occurring at similar rates in the isolated systolic hypertension and total population. The authors of the study concluded that the fixed-dose irbesartan/HCTZ combination treatment was effective and well tolerated in a diverse population of patients with isolated systolic hypertension.26
Although ACE inhibitors and ARBs, when used as monotherapy, often have diminished BP-lowering results in African-Americans, the addition of a thiazide diuretic blunts any across-group racial differences.71 One of the strengths of the INCLuSIVE trial was the large, heterogeneous population studied. Overall, in Caucasians, African-Americans, and Hispanics/ Latinos completing placebo treatment, mean SBP changes from baseline (placebo treatment end) to week 18 were -21.5±13.8mmHg for Caucasians, -20.7±16.5mmHg for African-Americans, and -22.9±13.2mmHg for Hispanics/Latinos (p<0.001 for each), and similarly among racial/ethnic subgroups.71 By week 18, 70% (95% CI 66–74%) of Caucasians, 66% (95% CI 59–74%) of African-American, and 65% (95% CI 57–74%) of Hispanic/Latino patients achieved the dual SBP/DBP goal. Therefore, irbesartan/HCTZ treatment provided SBP/DBP goal attainment in approximately two-thirds of Caucasian, African-American, and Hispanic/Latino patients with SBP that was uncontrolled on an antihypertensive.71 The overall incidence of AEs among the populations participating in the INCLuSIVE trial was similar across racial groups. In Caucasians, the incidence of any AEs in the placebo, hydochlorothiazide alone, and irbesartan/HCTZ 150mg/12.5mg and 300mg/25mg groups was 25, 17, 29, and 26%, respectively (AEs were mainly dizziness); in African- Americans these frequencies were 25, 19, 26, and 28%, respectively (AEs were mainly headache, constipation, upper respiratory tract infection, and pain in the extremities); and for Hispanics/Latinos the frequencies were 17, 12, 22, and 21%, respectively (AEs were mainly nasopharyngitis and dizziness). These AEs were predominantly transient and of mild to moderate severity.
An additional analysis of the INCLuSIVE trial showed the BP benefit of irbesartan/HCTZ fixed combinations in the subgroup ≥65 years of age with uncontrolled SBP, with SBP goal attainment of 73% in 212 patients ≥65 years of age with placebo (for four to five weeks), HCTZ 12.5mg (for two weeks), irbesartan/HCTZ 150mg/12.5mg (for eight weeks), then irbesartan/HCTZ 300mg/25mg (for eight weeks).27 From baseline to week 18 (n=184, intent-to-treat population), the mean change in SBP was -23.0±13.3mmHg and in DBP was -10.9±7.7mmHg, with mean SBP/DBP at study end of 134.0±14.7/75.1±8.4mmHg. In general, ARBs, including irbesartan, are well tolerated, with no increase in AEs or symptomatic side effects with the maximum approved doses. Furthermore, HCTZ appears well tolerated at 25mg and, when combined at the low- or high-dose fixed combination, there was increased BP lowering with minimal side effects.27
Examples of clinical studies using other ARBs (candesartan, eprosartan, losartan, olmesartan, telmesartan, and valsartan) in combination with other medications in the treatment of mild to moderate hypertension are summarized in Table 4. In most of these studies, combining the ARB with another treatment—in particular the diuretic HCTZ or the calcium channel blocker amlodipine—resulted in significant improvements in efficacy compared with ARB therapy alone. The studies demonstrate reductions in DBP, SBP, and overall control of hypertension in some cases in patients who were not controlled on ARB monotherapy.26,72,73 These combination regimens were all well tolerated and resulted in limited AEs—mainly headaches and fatigue. In the case of amlodipine, combining it with valsartan or telmesartan74,75 reduced the incidence of peripheral edema, which is a common problem with this drug. These studies represent a substantial body of experience with these drugs and show that all of them can be effectively used in combination with other drugs that are frequently used in hypertension treatment.
Diabetic Nephropathy
ESRD is primarily due to type 2 diabetes, usually comorbid with elevated BP, and its prevalent in the uS is continuing to rise. BP control is mandatory in order to decrease and perhaps reverse the progression of renal dysfunction. When prescribed earlier in the progress of diabetic nephropathy, irbesartan may also protect against progression of disease.
One of the clinical benefits of irbesartan is protection against the rate of progression of nephropathy in patients with type 2 diabetes and hypertension. The Irbesartan Diabetic Nephropathy Trial (IDNT) compared irbesartan versus the CCB amlodipine and placebo in 1,715 patients with type 2 diabetes and hypertension (SBP >135mmHg or DBP >85mmHg).30 Patients were also entered into the study if they demonstrated nephropathy (serum creatinine 1.0–3.0mg/dl in females and 1.2–3.0mg/dl in males, and proteinuria ≥900mg/day). The study population was 66% male and 72% Caucasian, and the mean age was 58.3–59.3 years. The majority of patients had mild to moderate hypertension with mean SBP values of 158–160mmHg and mean DBP of 87mmHg. Additional antihypertensive agents were permitted but could not include ACE inhibitors, other ARBs, or CCBs. The mean serum creatinine was 1.7mg/dl and the median protein excretion was 2.9g/day. Patients were followed up for a mean duration of 2.6 years. For the primary composite end-point (time to occurrence of doubling of serum creatinine, ESRD, or death), irbesartan, with additional antihypertensive therapy as needed, produced a 20% reduction compared with placebo (p=0.02) and a 23% risk reduction compared with amlodipine (p=0.006).30 The risk of doubling serum creatinine concentration was 33% lower in the irbesartan group than in the placebo group (p=0.003) and 37% lower in the irbesartan group than in the amlodopine group (p<0.001). Similar reductions in BP were observed with irbesartan 300mg versus amlodipine.
The Irbesartan in Patients with Type 2 diabetes and Microalbuminuria (IRMA 2) study was a multinational, randomized, double-blind, placebocontrolled study including 590 hypertensive patients with type 2 diabetes and microalbuminuria who were given irbesartan doses of either 150 or 300mg daily or placebo, and were followed for two years.31 The results showed that irbesartan has a significant dosedependent effect on microalbuminuria. The primary end-point of time to onset of diabetic nephropathy (persistent urinary albumin excretion rate >200μg/min and at least 30% higher than baseline) was reached in 10 of 194 patients in the 300mg group (5.2%) and in 19 of 195 patients in the 150mg group (9.7%) compared with 30 of 201 patients in the placebo group (14.9%) (p<0.001 and p=0.08 for the two irbesartan groups, respectively). Mean SBP during the study was significantly lower for the combined irbesartan groups compared with placebo (p=0.004) and serious AEs were less frequent with irbesartan. It was concluded that irbesartan has a renoprotective effect that is independent of its BP-lowering effect in patients who have type 2 diabetes with microalbuminuria.
After considering multiple similar studies, the JNC 7 concluded that there is a compelling case for using ARBs as alternatives to ACE inhibitors in patients with a combination of hypertension and type 2 diabetes and renal disease.41
Various studies have shown the other ARB medications to have renoprotective properties. In the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial, 1,513 individuals with type 2 diabetes and overt nephropathy were randomized to losartan (50–100mg once daily) or placebo, in addition to receiving conventional antihypertensive treatment for a mean duration of 3.4 years.76 Losartan provided significant benefits compared with placebo. For the primary end-point, losartan treatment reduced the composite of a doubling of the baseline serum creatinine concentration, ESRD, or death in 327 patients in the losartan group compared with 359 in the placebo group (16% risk reduction; p=0.02). For secondary end-points, losartan reduced the incidence of a doubling of the serum creatinine concentration (25% risk reduction; p=0.006) and ESRD (28% risk reduction; p=0.002), but had no effect on the rate of death (p=0.88). This effect was greater than would be expected from changes in BP alone. With losartan, proteinuria was reduced by 35% compared with placebo (p<0.001). Losartan treatment was also generally well tolerated. A further analysis of 1,428 patients in the RENAAL study showed that albuminuria reduction was associated with a lower risk of ESRD. In this study there was a lack of albumunuria reduction in 26% of the losartan-treated patients and 51% of the placebo-treated patients, demonstrating the renoprotective effects of losartan, in addition to BP reduction.77
The MicroAlbuminuria Reduction With VALsartan (MARVAL) study investigated the BP-independent effect of valsartan on elevated urine albumin excretion (uAER) in patients with type 2 diabetes with microalbuminuria.78 A population of 332 patients with type 2 diabetes and microalbuminuria, with or without hypertension, were randomized to 80mg/day valsartan or 5mg/day amlodipine for 24 weeks. There was a highly significant difference between patients treated with valsartan compared with those treated with amlodipne in uAER, the primary endpoint, at 24 weeks. For valsartan, uEAR was 56% (95% CI 49.6–63.0) of the baseline level and with amlodipine it was 92% (95% CI 81.7–103.7; p<0.001). The reduction in uEAR in valsartan-treated patients was similar in both the hypertensive and normotensive subgroups. In addition, more patients achieved normoalbuminuria with valsartan than with amlodpine (29.9 versus 14.5%; p=0.001). It was concluded that for similar degrees of BP reduction, valsartan lowered uAER more effectively than amlodipine in patients with type 2 diabetes and microalbuminuria, including the patients with baseline normotension. This indicates a BP-independent antiproteinuric effect of valsartan.
Renoprotection in diabetic nephropathy was also investigated in a double-blind, randomized, cross-over trial in 23 hypertensive patients with type 2 diabetes treated with placebo or 8, 16, or 32mg candasartan once daily over four treatment periods each lasting for two months.79 Reductions in albuminuria compared with placebo were 33, 59, and 52% for the 8, 16, and 32mg doses, respectively, the reduction with the two higher doses being significantly greater than that achieved with the lower dose and placebo. Glomerular filtration rate (GFR) was reduced by approximately 6ml/min/1.73m2, but there were no significant differences between doses in terms of reductions in BP. More recently, the large-scale Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study has investigated the efficacy of olmesartan in preventing or delaying the onset of microalbuminuria in patients with type 2 diabetes with normal albuminuria and at least one CV risk factor.80 Initial results show that 4,449 patients were enrolled and randomized to olmesartan 40mg daily or placebo and were monitored for a mean of 3.2 years. For the primary end-point, olmesartan demonstrated a 23% risk reduction in the development of microalbuminuria compared with placebo (p=0.0104). However, after adjustment for BP differences there was no significant difference between olmesartan and placebo. There was no difference between olmesartan and placebo in the secondary end-point of renal morbidity, (doubling of serum creatinine and ESRD); however, olmesartan therapy was associated with a reduction in estimated GFR (eGFR) of 4.76ml/min/1.73m2 compared with placebo (p<0.0001). The was no difference between olmesartan and placebo in terms of overall CV morbidity and mortality despite a significant higher number of CV deaths with olmesartan (n=15 versus n=3; p=0.0115). Treatmentemergent AEs were similar in terms of type and incidence in both the olmesartan and placebo groups. Overall, these and other studies highlight the benefit of all ARB medications in improving outcomes in diabetic nephropathy by either reducing the risk of progression to ESRD or reducing existing symptoms and limiting renal damage.
Conclusion
Antihypertensive medication is a vital part of treatment for patients with mild to moderate hypertension, a disease that encompasses pre-hypertension, stage 1 hypertension, and lower levels of stage 2 hypertension. In patients with this degree of hypertension, irbesartan is a safe and effective antihypertensive agent that can help prevent long-term organ damage, particularly to the kidneys, which often leads to ESRD.
There is now a substantial body of clinical trial data showing irbesartan to be effective in reducing DBP and SBP and establishing control of mild to moderate hypertension in large patient populations including diverse racial groups, older age groups, obese patients, and patients with systolic hypertension or heart failure. In several studies irbesartan and, indeed, other ARBs showed increased efficacy when combined with other antihypertensive medications, particularly the diuretic HCTZ and the CCB amlodipine. In addition, irbesartan and other ARBs have been shown to decrease proteinuria and delay the progression to ESRD in patients with diabetic nephropathy.
The low incidence of AEs and marked efficacy in controlling blood pressure therefore make irbesartan suitable for use either alone or in combination with other medications for the treatment of a diverse range of patients with mild to moderate hypertension.