Endovascular techniques for renal denervation (RDN) as a treatment for hypertension were initially highly encouraging with reductions in blood pressure (BP) in the range of 30 mmHg.1 However, these high expectations received a major setback following the unexpected results of the sham-controlled Renal Denervation in Patients With Uncontrolled Hypertension (SYMPLICITY HTN-3) trial. This promising procedure was faced with a major roadblock and, in general, the expectations that this would stand the test of time were markedly tempered.2 Like all good trials, SYMPLICITY HTN-3 provided answers, but also generated new questions, particularly in regard to procedural and technical issues. This, in turn, has led to a series of new and ongoing trials, which hopefully will resolve the issue of whether this procedure will become a routine aspect of hypertension management. In the present article, we review the literature in support of the RDN concept as well as emphasize the potential pitfalls and possible future direction of RDN.
Proof of Concept: A Historical Perspective
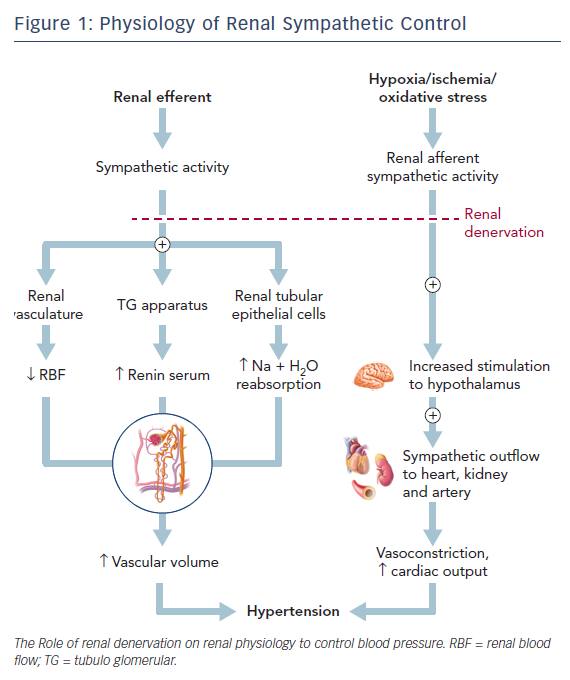
The renal system, through the maintenance of fluid–electrolyte balance and its interplay with the autonomic nervous system, plays an essential role in the pathophysiology of hypertension. Increased sympathetic nervous system activity as a cause and result of hypertension has been proven in various human experiments.3 The activation of the efferent sympathetic system via the postganglionic sympathetic neuronal chain relayed to the kidneys over the renal vasculature results in one or all of three effects; increased renin secretion from the juxtaglomerular apparatus; renal vasoconstriction decreasing renal blood flow; and enhanced sodium and water absorption. This contributes to an increased intravascular volume and arteriolar vasoconstriction adding to hypertension.4,5
Renal structures are also richly innervated with baroreceptors and chemoreceptors. Stimulation of these nerves by metabolites like adenosine, which form under conditions of ischemia and oxidative stress, result in increased input into the hypothalamus augmenting the sympathetic outflow, not only to the kidneys, but also to other structures like the heart and peripheral arteries, resulting in neurogenic hypertension.5–7 Interruption of this neural traffic underlies the concept of RDN for the treatment of hypertension. Esler et al. demonstrated increased renal sympathetic nervous activity in adults with essential hypertension. His assessment of renal norepinephrine spillover using isotope dilution measurements showed elevated levels in comparison to normal individuals.8 Also, this increase was higher in young adults (<40 years) with essential hypertension compared to older individuals. On comparison of 34 patients with essential hypertension and 23 normal patients, Esler et al. demonstrated than in those with hypertension, plasma concentration of noradrenaline and rate of release of noradrenaline into plasma was 32 % and 38 % higher, respectively, than in normal individuals.9 Schlaich further demonstrated a sustained decrease in BP from 161/107 to 127/81 mmHg at 12 months following bilateral renal artery ablation (Figure 1).10
One of the earliest treatment modalities for hypertension preceding the use of medications was surgical RDN. Smithwick and Thompson followed up 1,266 cases of thoracolumbar sympathetic splanchnicectomy over a minimum of 5 years. They compared the management of hypertension in these patients with that of in 467 medically treated controls to assess the mortality and efficacy of surgery as treatment for hypertension.11 Sympathetic splanchnicectomy decreased all-cause mortality in contrast to medically treated controls. However, this non-selective sympathectomy technique resulted in very severe debilitating side-effects, including orthostatic hypotension, palpitations, peripheral vasoconstriction, gastrointestinal dysfunction and sexual dysfunction as varying accompaniments. The introduction of the newer potent antihypertensive drugs soon drove surgical denervation into near extinction given the risk of potential complications as well as the efficacy of the newer medications.12,13
Catheter-based Renal Denervation
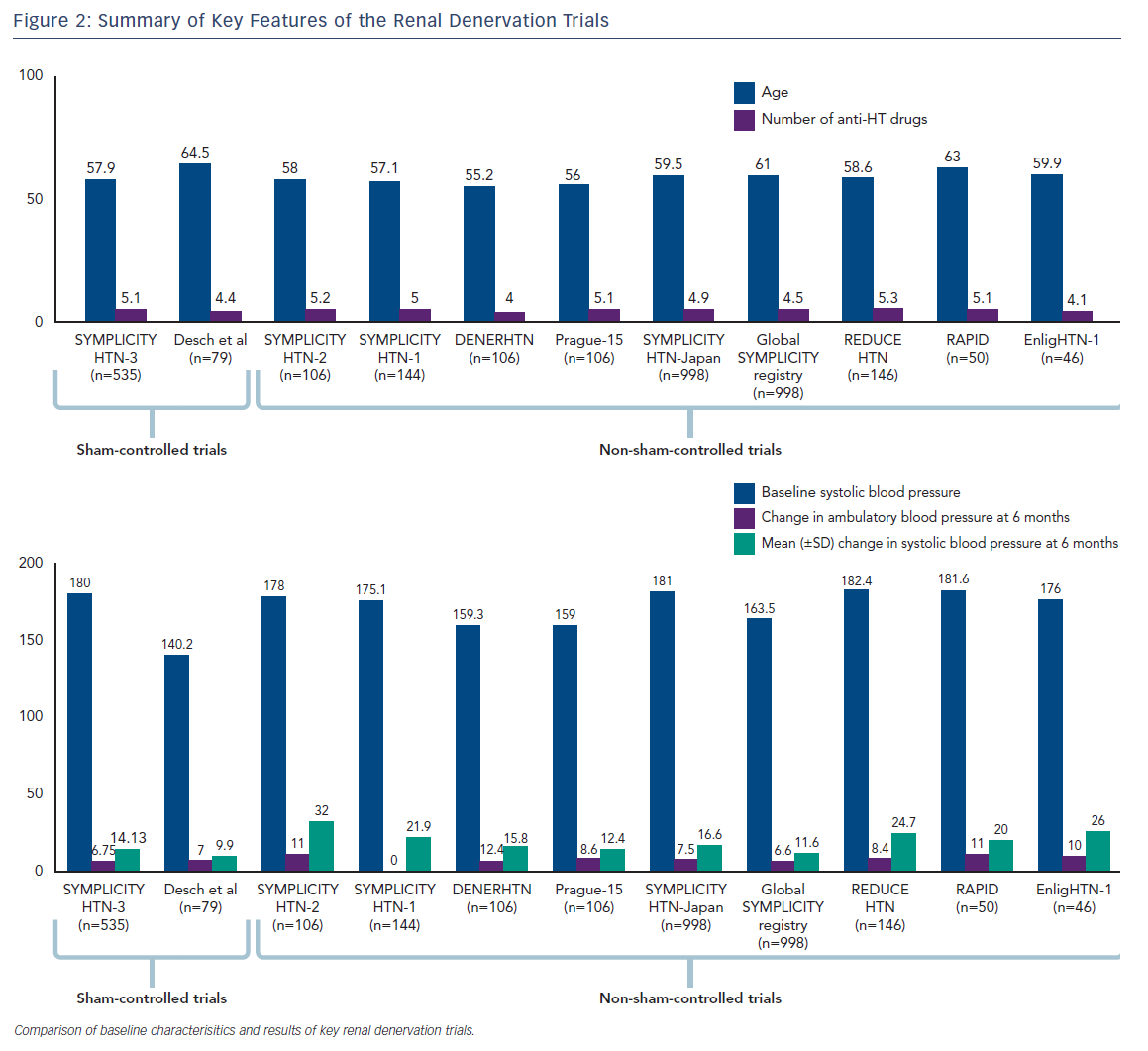
Ablation using radiofrequency (RF) has been the subject of multiple trials (Figure 2) using different catheter-based systems among which the majority used the SYMPLICITY RDN catheter (Medtronic Inc). This had unipolar platinum-iridium electrodes at the tip and the catheter was introduced percutaneously into the renal artery.14 Multiple (four to six) RF ablations of 8 W for up to 2 minutes applied in a helical manner to the arterial endothelium were used to ablate the sympathetic nerves; this was then replicated in the other renal artery to achieve RDN. Measures to confirm the completeness of this procedure were variably used.
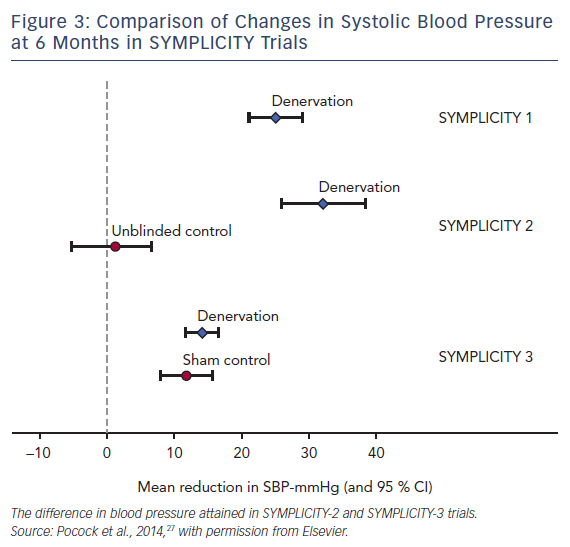
SYMPLICITY HTN-1, published in 2009, was the first trial to establish that catheter-based RDN reduced BP (−27/−17 mmHg at 12 months) in patients with rHTN with a reduction of sympathetic activity measured by renal noradrenaline spillover.1
Considering the major, although not unexpected, critical limitation of the study, like the absence of the control group, a new randomized prospective, single-blind, multicenter trial called the SYMPLICITY HTN-2 was initiated.15 The impressive positive results (–32/–12 mmHg) led to great enthusiasm for the procedure (Figure 3).
However, a reality check in the form of the results of the SYMPLICITY HTN-3 prevailed. This sham-controlled trial designed to adjust for the effects of a sham procedure showed no significant difference in BP between the target groups at the end of 6 months. The difference in BP between the denervation group compared with the sham-procedure group was only −2.39 mmHg, which was not statistically significant (p=0.26).14 The unexpected results of a trial that was presumed to provide a definitive statement led to a number of trials being discontinued and several possibilities continue to be considered as to why the results were disparate in comparison to the earlier trials.16
Renal Denervation for Hypertension (DENERHTN) was an open-label, prospective, randomized trial that compared RDN to a standardized stepped-care antihypertensive treatment with a blinded endpoint evaluation in patients with rHTN performed in 15 tertiary centers in France.17 However, in comparison to the SYMPLICITY trials, the primary endpoint was the ambulatory BP. Both groups showed a reduction in daytime ambulatory systolic BP, with a greater significant reduction in the RDN group (−15.8 mmHg versus −9.9 mmHg; p=0.0329). However, the small change in BP compared to non-sham controlled groups did not do much to alleviate the fears that RDN had failed as the results of the SYMPLICITY HTN-3 implied.
Limitations of Current Techniques: Behind the Reality Check?
The techniques used in various trials as well as the ones in SYMPLICITY HTN-3 trial had various shortcomings.
Variation in Renal Anatomy
Histological assessment of the renal vessels at autopsy demonstrated that the maximum mean nerve density was observed in the proximal and middle segments of the renal artery, whereas the least average number of nerves was seen in the distal segment. However, the nerves converge in proximity toward the distal renal arteries and thus the energy delivered by the ablation catheter is most effective here. The mean distance from the lumen to nerves was lowest in this region.18 Further variability in the form of accessory renal arteries with their smaller caliber makes it difficult to ablate within this vessel. Current endovascular techniques of ablation may not account for these anatomical changes thereby partly explaining the variability in results.19 The SYMPLICITY HTN-3 trial was also affected, as the energy delivered by the catheter was concentrated on the proximal renal arteries as per protocol.
Non-contiguous Lesions
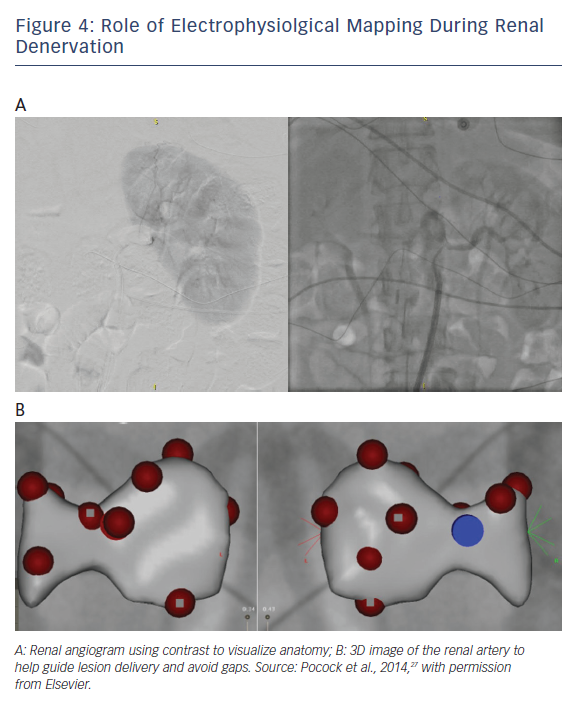
The anatomical separation between the lumen of the vessel and the adventitia (containing nerves) is also variable along the length of the vessel with the thinnest wall being present distally (50th percentile values = 1.81 mm distally) causing the nerves to converge on the vessel near the renal hilum. Therefore, there is the need for a transmural lesion creation over a minimum depth of 2 mm.19 This, accompanied by a wide variation in nerve distribution patterns and an inability to map electrical nerve signals necessitates a circumferential ablation technique for RDN. While the site of the ablation could be debated, continuity and transmurality need to be present, like the lesions needed to isolate pulmonary veins in AF. This may necessitate the use of electrophysiological mapping systems to ensure the absence of gaps when creating transmural lesions in addition to use of imaging techniques like intracardiac echo to view lesion formation at the time of ablation (Figure 4).
Improper Energy Delivery
The renal artery is near the renal vein distally and to the para-aortic vessels proximally. RF energy delivery depends on resistive heating to cause lesions. The presence of these vessels increases the washout of thermal energy created in the local region during RF energy delivery. The inadequate lesions created due to the washout of thermal energy maybe responsible for the limiting results.
Limitations of Radiofrequency Energy Delivery
Radiofrequency energy causes thermal damage to the endothelial tissue and carries with it the potential of char and coagulum when delivered using non-irrigated catheters and stem pops when delivered using irrigated catheters. Studies using optical coherence tomography have demonstrated endothelial damage at sites of ablation.20 Given that distal vessel lesions may be of potential benefit in achieving neural damage, the use of alternative energy sources that may have a lesser propensity of endothelial damage may be useful. In addition, the more distal the lesion, the greater the chances for complete vessel occlusion, thereby necessitating avoidance of endothelial damage as much as possible.
Absence of an Endpoint for Procedural Efficacy
A lack of procedural endpoint has been a limitation in several studies. In addition, norepinephrine spillover has not been a consistent procedural endpoint to evaluate the procedure. Krum et al. used this to evaluate the efficacy of their denervation, which correlated with the long-term reduction in the BP over 6 months.21 However, this extremely time-consuming and laborious process cannot be a used on a routine basis clinically.
Absence of a procedural endpoint is an Achilles heel, specifically to the SYMPLICITY HTN-3 trial where the proceduralist had no prior experience with RDN technique.14 De Jong et al. proposed that the difference between acute renal nerve stimulation-induced BP rise before and after RDN is a significant predictor of outcome after RDN.22 Gal et al. reported an acute, temporary rise in BP with intrarenal electrical stimulation of the renal artery, which was significantly blunted following RDN. This, however, has not been widely investigated and is limited by the need for general anesthesia as renal nerve stimulation causes pain.23 The design of a catheter in the future that can potentially carry out both stimulation as well as ablation as used for the ablation of arrhythmias could be helpful.
The recording of electrograms from renal sympathetic nerves which could record bursts of renal nerve activity in the periarterial nerves has also been hypothesized as a technique for assessing the success of denervation procedure.24
Proceduralist Learning Curve
Much has been written about the effects of the proceduralist learning curve on the efficacy of the RDN procedures. Potentially better results may be expected in more experienced hands.25
Patient Selection
Patients with hypertension are the targets in whom the procedure is thought to deliver substantial benefit. However, given that the etiology is multifactorial, the response may be varied. A pooled data of 1103 patients from SYMPLICITY HTN-3 and the Global SYMPLICITY Registry showed the reduction in BP among patients with isolated systolic hypertension was less pronounced than the reduction in patients with combined hypertension. Ewen et al. also demonstrated similar effects in reduction of BP in 126 patients undergoing bilateral RDN with resistant HTN.26 This could be possibly explained by the less-pronounced sympathetic activity in older patients with isolated systolic hypertension. Also, as isolated systolic BP is characterized by stiffness of the arteries with increased pulse pressure, the hypothesized concept behind RDN would not be applicable. This in fact could partly explain the subsets in SYMPLICITY HTN-3 where patients aged <65 years tended to respond better to RDN when compared to patients aged >65 years.14
Placebo Effect, Regression to the Mean, and the Hawthorne Effect
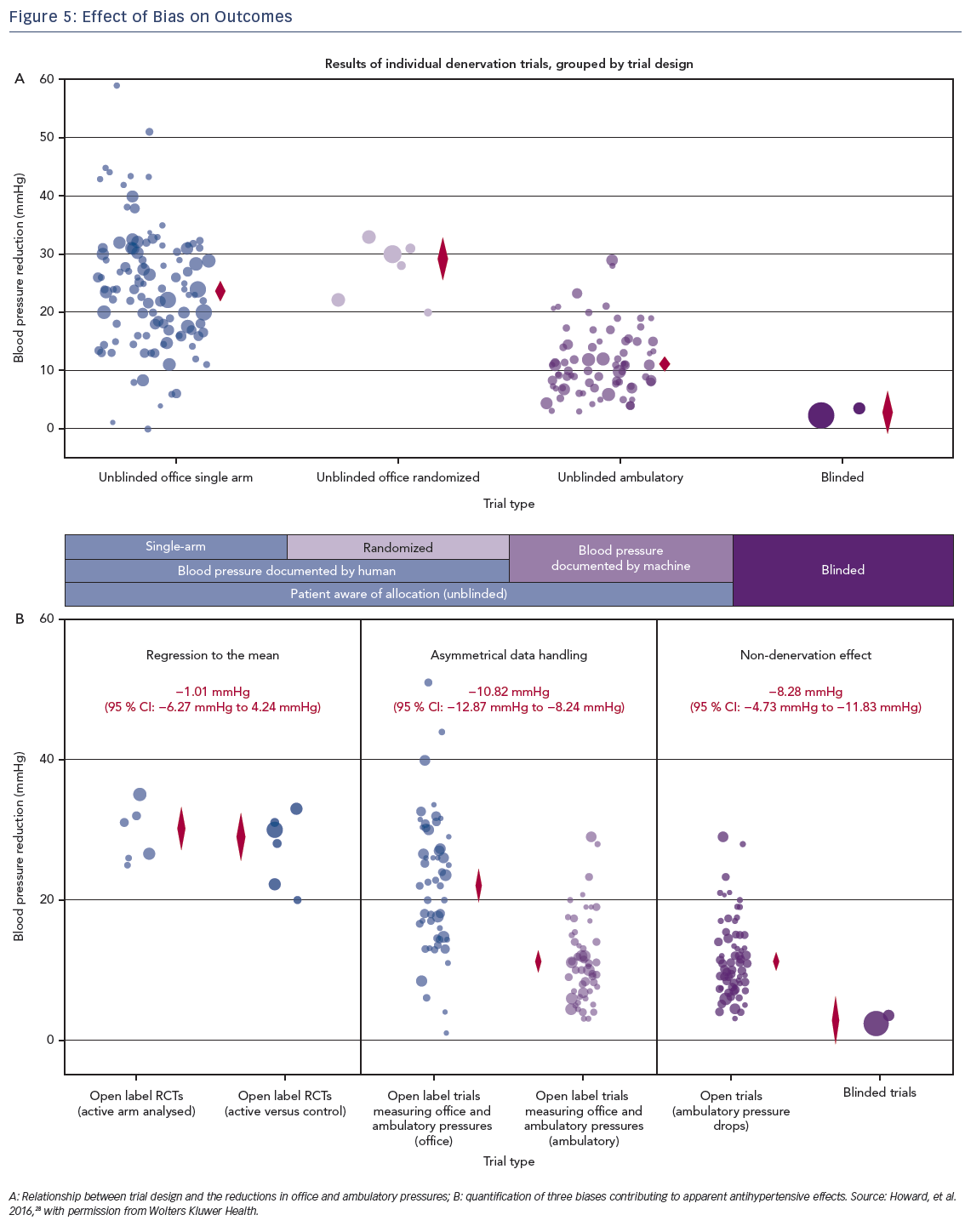
The powerful effect of placebo cannot be ruled out given that the initial trials did not compare endovascular RDN against sham procedures. Researchers in the early SYMPLICITY trials and the DENERHTN trial used physician-based BP measurements and automated 24-hour BP measurements, respectively, as endpoints for the trials. Despite these efforts, there were no effective means to counter the bias arising from a regression to the mean (RTM). Since enrollment into trials is when there is patient contact with the caregiver, it is likely that the initial contact was made at the height of the patient symptoms and primed for variability orfall. Pocock et al. using analysis of covariance demonstrated that RTM was found in both the RDN and sham control arms of SYMPLICITY-3 HTN trial, but was not responsible for the neutral findings.27 To estimate if this change is secondary to the therapeutic procedure or merely a variation, it is important to have a randomized control group. Since none of the initial trials were designed to accommodate for this bias, it is likely that there must have been some contribution of the same to the impressive results.14 Howard et al. conducted a meta-analysis of the various randomized and non-randomized trials of RDN to study the disparate results and demonstrated that the disparity may be due to asymmetrical data handling and confounding which can only be reduced by randomization and importantly blinding28 (Figure 5a,b). However, in SYMPLICITY HTN-3, despite accounting for this there was a neutral trial result in both the groups.
The observer effect, or the Hawthorne effect, which describes the change in the behavior in subjects when under observation, accounts for some of the improvement seen in the placebo arms of trials. A potential ‘reverse Hawthorne’ effect was noted in the SYMPLICITY HTN-2 trial. Patients anxious to undergo RDN after having known about the results of the initial SYMPLICITY trial may have had reduced compliance with medications. This remains open to speculation. However, a urine analysis-based prospective evaluation of adherence to prescribed medications did show a decrease in compliance in a group of patients who underwent RDN. This noncompliance was maximal for the vasodilator class of medications.29
Ethnic Considerations
There was a difference between population cohort recruited to the initial SYMPLICITY trials and the SYMPLICITY HTN-3 trial. Although underpowered subgroup analyses of the fall in BP in the non-African-American cohort showed a difference, these findings remain hypotheses generating. In addition, the large magnitude changes seen within the early trials were absent.30
SPYRAL HTN-OFF MED Trial
The SPYRAL HTN trial with two arms to assess the efficacy of the procedure alone (SPYRAL HTN OFF-MED) and in comparison, to antihypertensive drugs (SPYRAL HTN ON-MED) aimed to address these concerns.31 These were designed to be proof-of-concept trials to demonstrate the ability of RDN to influence BP in uncontrolled hypertension.
Twenty-one recruiting sites across the world were used to recruit 80 patients randomized to RDN with a new SPYRAL catheter (n=38) or a sham procedure (n=42) in the SPYRAL HTN-OFF MED arm of the study. Patients were followed up to 3 months and the primary endpoint was the change in 24-hour BP at the end of the follow-up period. Drug surveillance was done to ensure patient compliance with the absence of anti-hypertensive medication.
The differences in the study design between the SYMPLICITY-3 trial and the SPYRAL HTN-OFF MED arm included the absence of anti-hypertension drugs at the time of the randomization, the inclusion of patients with moderate hypertension, the exclusion of patients with isolated systolic hypertension and the use of a new catheter designed to ablate distally within the branches of the renal artery. Highly experienced operators were involved in conducting and proctoring the trial. In addition, a standardized approach based on advances in the understanding of renal nerve anatomy to target the renal artery along with its accessory branches was followed.
A blinding index of 0.65 (CI [0.56–0.75]) at discharge and 0.59 (CI [0.49–0.70]) at 3 months after the procedure was attained. Near 60 % of the total ablation lesions in the RDN group were delivered to the branch vessels within the kidney and 88.6 % patients did not have any anti-hypertension drugs at baseline and at 3 months in the RDN group (82.9 % in the sham group). When reviewed at 3 months, a significant difference (−5/4.4 mmHg; p-value 0.04/0.002) was noted on the 24-hour ambulatory BP monitoring between the RDN and the sham control groups. Similar significant differences between the two groups were also noted on the office BP recordings (−7.7/−4.9 mmHg; p=0.02/0.008) with the safety results suggesting no complications from the procedure.
The results of the SPYRAL HTN-ON MED arm purported to obtain an assessment of the efficacy and safety of RDN in the presence of three standard antihypertensive medications are still awaited (NCT02439775). This is a sham-controlled, randomized, parallel assignment, single blinded, treatment driven trial, having safety outcomes at 36 months post procedure and change in the systolic BP measured by 24-hour ambulatory BP monitoring as the primary outcomes.31,32
Future Directions
Selective Versus Broad Denervation
Deciding between ablation of nociceptive afferent fibers selectively versus total ablation including efferent fibers is important intra-procedurally given that their proximity to the adventitia varies as the distance from the aorta varies.19 In case of selective afferent fiber damage, proximity to the aorta is beneficial for the ablation, whereas for the efferent fibers, ablation needs to be performed more distally. It is unclear whether selective afferent fiber damage is beneficial for antihypertensive effect, particularly given that centrally acting medications do not decrease the BP more than what has been seen in patients undergoing RDN.33 Hedging the bets on one or the other is the current paradigm but an answer is still elusive.
Procedural Efficacy Endpoint
Despite the promising results of the recent trials, there remains the looming question of evaluating the procedural efficacy acutely. Given the inability to use norepinephrine spillover values acutely in a routine clinical setting, the search for the endpoint remains ongoing. Efforts to use an electrogram-based endpoint need further data before they can be used. In addition, translational research pertaining to the provocative maneuvers targeting afferent sympathetic nerves to evaluate if there is an acute response to the procedure needs further development and human validation. Inability to find this piece of the puzzle is a major stumbling block in current technology.
Prediction of Response
There remains wide variability in the response to the RDN procedure. Even in the SPYRAL HTN trial, there was a significant drop in BP in some with no response in the others. Evaluation of the same to find predictors of response remains a focus of investigation to better identify patients who can benefit from this technology.
Sympathetic Re-innervation
Denervation is mainly an axonotmesis, not neuronotmesis. Potential sparing of the autonomic sympathetic ganglia allows for the possibility of re-innervation. Data from porcine and ovine models lends support to this hypothesis.34,35 This has always been found to be incomplete and patchy. Similarly, data from other denervation models in humans (post cardiac transplantation) have always shown incomplete neural re-innervation and limited functionality leading to skepticism of its contribution in the long term.36 The results of The Australian SHAM Controlled Clinical Trial of Renal DeNervation in Patients With Resistant Hypertension (AUSHAM-RDN-01) study are awaited to shed some more light on this matter.
Alternate Energy Sources
Given the potential shortcomings of RF energy, cryoenergy has been evaluated in animal models and has found some success. However, it remains investigational without any human clinical data at present.37
The PARADISE catheter system uses a non-contact catheter system emitting sound waves circumferentially to cause sympathetic nerve damage via their conversion to thermal energy. Preliminary results in the REDUCE HTN trial using PARADISE catheter system were comparable to published data on RF RDN with a mean reduction in office and home BP of −36/−17 mmHg and −22/−12 mmHg, respectively.38 The Study of the ReCor Medical Paradise System in Clinical Hypertension (RADIANCE HTN) is a sham-controlled, randomized, double-blind study currently evaluating this concept in human trials.
Due to the thermal nature of tissue destruction with both these modalities, there are still challenges, such as endothelial damage and heat sink phenomenon.
Application of direct current energy in varying pulses can be used to cause tissue destruction via electroporation.39,40 As the mechanism of tissue damage is via the formation of large pores within the cell membrane causing apoptotic cell death, it is unlikely to be affected by the heat sink phenomenon. Coagulum formation leading to distal renal embolization and local inflammation are challenges that may not be avoided by use of this energy source. In addition, the virtual electrode properties of the electric field make it very attractive for use given the wide variability in the anatomy of the nerves. Non-selective neural ablation may also be facilitated. However, this needs evaluation for efficacy and feasibility.
Use of capsaicin via micro-needles, and use of guanethidine to cause sympathetic destruction via an immune mechanism are still experimental methods which need evaluation.41,42
Non-endovascular Techniques
Whereas most of the efforts are directed towards endovascular RDN, there remains a niche for the use of minimally invasive techniques for the delivery of neurotoxic substances for RDN. Laparoscopy as a technique for RDN for the treatment of renal hypertension has not been studied well enough. However, the number, three-dimensional arrangement, and location of the peri-arterial nerves were the main limiting factors when laparoscopic RDN was tried for intractable polycystic kidney disease pain.43 These are also limited by the need for local fat dissection, inadequate delivery methods, intra-abdominal adhesions and possibly longer recovery times.
Other Benefits for Renal Denervation
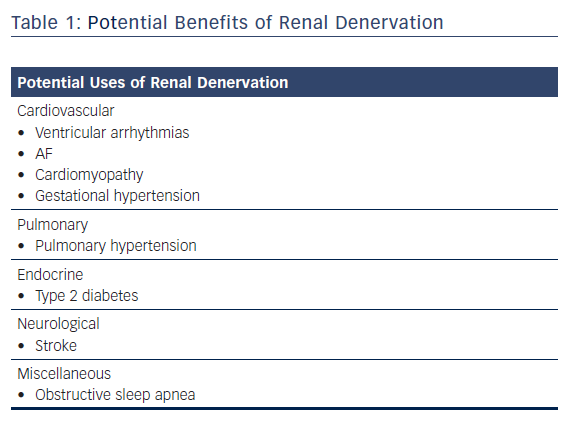
Besides the obvious benefits on the management of hypertension, RDN also has the potential to offer other systemic benefits that are purely investigational at its present stage (Table 1).
Managing Arrhythmias
Premature Ventricular Contraction-mediated Cardiomyopathy
Renal denervation inhibits the development of cardiac fibrosis and the risk of VF by inhibiting the renin angiotensin aldosterone system (RAAS) and sympathetic activity. Yamada et al. demonstrated RDN has a protective effect on the development of left ventricular enlargement and biventricular fibrosis in rabbits with high PVC burden.44
Ventricular Tachycardia Storm
Following RDN, ventricular tachyarrhythmias were significantly reduced in two patients with therapy-resistant electrical storm.45
Atrial Arrhythmias
Linz et al. demonstrated attenuation in the incidence and duration of spontaneous AF on RDN in an obstructive sleep apnea (OSA) model, which was comparable to pharmacological therapy.46 Hou et al. also showed a similar effect of RDN in reducing AF inducibility and reversing the atrial electrophysiological changes induced by sympathetic hyperactivity.47
Reduction in Stroke Risk
RDN was shown to decrease the incidence of stroke in hypertensive rats, not just by the reduction of BP, but by the attenuation of oxidative stress and inflammatory effects in brain, the improvement of cerebral blood flow and the inhibition of the blood brain barrier disruption. The suppression of RAAS has shown to decrease the incidence of stroke independent of the BP as angiotensin II alone is involved in stroke. However, further studies are needed to evaluate this concept.48,49
Treatment of Type 2 Diabetes
The effect of RDN on glucose metabolism and insulin resistance was studied by Mahfoud et al. demonstrating a significant improvement.50 RDN in the management of T2DM can emerge a synergistic measure to medication perhaps by improving the sensitivity to circulating insulin. This bears evaluation in further studies.
Obstructive Sleep Apnea
Obstructive sleep apnea affects up to 80 % of patients with rHTN with OSA being considered as both a consequence and cause of increased sympathetic tone.51 In one study, RDN was shown to decrease the severity of OSA and attenuate apnea/hypopnea index, thus suggesting RDN as treatment for OSA.52
Pulmonary Hypertension
RDN by the attenuation of RAAS and sympathetic outflow was demonstrated to improve pulmonary vascular remodeling and reduce right ventricular afterload and decreased right ventricular stiffness.53
Gestational Hypertension
The enhanced pressor responses to angiotensin II by upregulation of mRNA expression of several RAAS components in offspring of rats with gestational hypertension is reversed by RDN.54
Cardio-renal Syndrome: Reduction in Renal Fibrosis
Chronic activation of RAAS resulting in increased sodium reabsorption has been linked to myocardial and renal fibrosis.55 RDN significantly decreased the sympathetic nervous system activity and RAAS, thereby lowering collagen synthesis biomarkers concentration in plasma and reduction of cardio-renal collagen volume fraction in histopathology in rats with isoproterenol-induced cardiomyopathy.56
Conclusion
Renal denervation remains a concept with a robust burden of surgical data backing its efficacy, the benefit of which remains lost in translation with the endovascular approach. It is important to continue investigating these techniques, particularly given the wide-ranging impact of this intervention on autonomic homeostasis, with due care given to accuracy and reproducibility of benefits. Recent trials like the SPYRAL HTN OFF-MED have indeed given hope that the paradise deemed to have been lost, may in fact have shown signs of being regained.