Percutaneous Coronary Intervention for Clinical Syndromes
Stable Angina
The primary benefit of percutaneous coronary intervention (PCI) over medical therapy in patients with stable angina is the improved quality of life. The PCI in stable angina (ORBITA) trial was the first sham-controlled trial of PCI where 200 medically optimized patients with single vessel disease were randomized to PCI or placebo procedure.1 The trial failed to show a benefit of PCI at 6 weeks in the primary endpoint of exercise treadmill time or secondary endpoints of patient-centered outcomes. Limitations of the trial included high exercise time and low ischemic burden at baseline and a short follow-up period.1 The effect of medications and the uncertainty of stent treatment may have also influenced exercise time. The landmark trial, however, showed similar short-term outcomes in both study groups, and its findings support shared decision making and a conservative approach in patients with stable angina and similar risk profiles to those in the trial.
Cardiogenic Shock
Holger et al. reported the 1-year outcomes of the randomized Culprit lesion only PCI versus multi-vessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial.2 The primary endpoint at 30 days favored the culprit-only approach, with significantly lower rates of the composite of death or renal-replacement therapy (45.9% versus 55.4%, p=0.01).2 At 1 year follow-up, there was a trend towards lower risk of the composite of death and recurrent infarction in the culprit-only group (relative risk 0.87; 95% CI: 0.76–1.00) that did not reach statistical significance. In addition, rates of repeat revascularization (32.3% versus 9.4%; relative risk 3.44; 95% CI [2.39–4.95]), and rehospitalization for heart failure occurred more frequently in the culprit-only group.2 The 1-year results support a culprit-only approach in the acute setting for MI with cardiogenic shock, however, when patients stabilize and their risk of acute kidney injury is lower, ischemia-guided or complete revascularization to prevent subsequent unplanned revascularization or admissions may improve long-term outcomes in this high-risk patient cohort.
Unloading the left ventricle (LV) with mechanical support before revascularization in acute ST segment elevation MI (STEMI) has also been under investigation. The theory is that unloading will limit infarct size and translate into lower mortality. The recent multi-center, prospective, randomized trial by Kapur et al. demonstrated feasibility of implantation of the Impella CP device (Abiomed) in STEMI with a mean longer door to balloon time of 25 minutes, with no difference in clinical outcomes or infarct size.3 A larger pivotal trial to compare pre-reperfusion unloading to the standard of care is needed before implementing a change in clinical practice.
Out-of-hospital Cardiac Arrest
The optimal timing of cardiac catheterization in patients with out-of-hospital cardiac arrest (OHCA) of presumed cardiac cause but without STEMI is unknown. The French Registry for OHCA evaluated the association between an immediate invasive strategy and survival in a cohort of 1,410 patients, according to their cardiac arrest hospital prognosis score.4 This score is calculated from: age, setting or arrest, initial rhythm, duration from collapse to basic life support and from basic life support to return of spontaneous circulation, pH and epinephrine dose.4 They observed higher rates of early coronary angiography in patients with better predicted prognosis (a low-risk score) and an overall survival rate at hospital discharge of 32%.4 An immediate invasive strategy was independently associated with better survival in low-risk patients (OR: 2.3, 95% CI [1.4–3.9]; p=0.001), but not in medium-risk (p=0.55) and high-risk (p=0.43) patients.4 This suggests that, until randomized data are available, immediate coronary angiography in OHCA upon arrival to the hospital should focus on patients with preserved neurological status.4
Coronary Lesion Subsets
Chronic Total Occlusions
New techniques and equipment has accelerated interest in chronic total occlusions (CTO). The CrossBoss First trial was a multicenter, randomized-controlled trial that compared upfront use of the CrossBoss microcatheter (Boston Scientific) versus standard wire escalation for antegrade CTO crossing.5 This study included 246 patients and showed no difference in procedural success, procedure time or equipment cost suggesting that either approach is reasonable. Werner et al. performed a randomized trial in 396 patients to assess symptomatic improvement with CTO PCI. Patients were randomized 2:1 to CTO PCI or optimal medical therapy.6 At 1 year there was a greater improvement in the patients’ Seattle Angina Questionnaire scores and they were free from angina with PCI (p=0.003).6 Additionally, those who underwent PCI were found to have an improved overall quality of life (p=0.007).6 This study suggests that an objective assessment of angina burden and quality of life in CTO patients may identify those with the greatest potential to benefit from revascularization.
Bifurcations
At the end of 2017, the Double Kissing Crush (DK Crush) V trial was published. This randomized trial of unprotected distal left main coronary artery (UPLM) bifurcation lesions compared a planned two-stent strategy (PS) with a double kissing crush technique versus provisional stenting in 482 patients.7 In order to participate, operators needed to demonstrate competence in the performance of a minimum of 3–5 DK crush cases and perform 300 PCIs per year for 5 years, including at least 20 LM PCIs per year.7 Target lesion failure at 1 year was significantly lower in the DK crush group (5.0% versus 10.7%; HR 0.42; 95% CI [0.21–0.85]; p=0.02).7 In the 13-month angiographic follow up, the rates of in-stent restenosis (ISR) in the main vessel was similar between the PS and DK Crush subgroups.7 However, the rate of ISR at the ostium of the side branch was 12% with PS versus 5.0% in the DK crush (p=0.09) group, further supporting the use of DK Crush for UPLM bifurcation lesions.7
Saphenous Vein Graft Interventions
Acute procedural complications and risk of target vessel failure of PCI for saphenous vein graft interventions (SVG) lesions has led interventionalists to consider native vessel over SVG PCI when feasible. In 2018, two studies were published that questioned the efficacy of drug-eluting stents (DES) compared with bare metal stents (BMS) for saphenous vein graft (SVG) interventions. The Drug-Eluting Stents vs. Bare Metal Stents In Saphenous Vein Graft Angioplasty (DIVA) trial, a double-blind, randomized trial at Veterans Affairs centers, focused on SVG PCI with planned embolic protection devices.8 Patients were randomized 1:1 to BMS or DES for de novo SVG stenosis of 50–99% in grafts 2.25–4.5 mm in diameter.8 Randomization was stratified by presence of diabetes and number of lesions.8 At 12 months, the incidence of target vessel failure was 17% in the DES group versus 19% in the BMS group (adjusted HR 0.92, 95% CI [0.63–1.34], p=0.70).8
The 5-year results of the Efficacy Study of Drug-Eluting and Bare Metal Stents in Bypass Graft Lesions (ISAR-CABG) study, which randomized 610 patients to first generation DES versus BMS for SVG stenosis, showed no difference in the primary endpoint of death, MI, or target lesion revascularization (HR 0.98, 95% CI [0.79–1.23]; p=0.89).9 Although the 1-year outcomes favored DES with significantly lower target lesion revascularization, there was a twofold higher risk during years one to five that negated the initial benefit.9 The mechanism of late DES failure is unclear but it is maybe due to inadequate suppression of neointimal hyperplasia or early neoatherosclerosis. Further investigation is needed to determine the optimal approach to PCI in patients with SVG disease. At present, PCI with either BMS or DES is an acceptable choice for SVG PCI, however, the studies were not powered to assess SVG subgroups in which DES may confer a benefit or harm. An additional consideration is to perform native vessel PCI for SVG failure when it is a feasible option.
Thrombus-containing Lesions
Primary PCI in STEMI is high risk due to thrombus and potential for no reflow. While earlier studies showed a benefit of manual thrombus aspiration on procedural outcomes, large randomized trials demonstrated that routine use of aspiration thrombectomy had no benefit on mortality. A meta-analysis by Jolly et al. evaluated the use of thrombus aspiration during primary PCI.10 Randomized controlled trials, including at least 1,000 patients comparing manual thrombectomy and PCI alone in patients with STEMI were included. Routine thrombus aspiration did not improve clinical outcomes. However, in the subset with high thrombus burden, the trend towards a reduction in cardiovascular death were balanced by an increased risk of stroke or transient ischemic attack.10 The meta-analysis supports the current guideline recommendation against routine use of aspiration in STEMI.11
Calcified Lesions
Calcified coronary lesions increase PCI complexity and complications. Intravascular lithotripsy (IVL) has recently been approved in Europe for severely calcified lesions. It uses sonic pressure waves locally to effectively fracture both intimal and medial calcium in the artery wall without damage to surrounding soft vascular tissue.12 The Shockwave Coronary Lithoplasty® (DISRUPT CAD II; NCT03328949) post-market study is currently recruiting in Europe, to study IVL technology in calcified coronary lesions.
In-stent Restenosis
The optimal management of DES in-stent restenosis (ISR) is controversial. The Restenosis Intra-stent of Drug-eluting Stents: Paclitaxel-eluting Balloon versus Everolimus-eluting Stent (RIBS IV) trial randomized patients with DES ISR to either a paclitaxel-eluting balloon (DEB) or everolimus-eluting stent (EES). At 3 years, cardiac death, MI and target lesion revascularization was significantly lower in the EES arm (12.3% versus 20.1%; p=0.04).13 However, the Drug-eluting bAlloon for in-stent Restenosis (DARE) trial, a non-inferiority study comparing DEB and DES for ISR (56% with DES-ISR) showed similar rates of target lesion revascularization at 12 months with DES (7.1% versus DEB 8.8%; p=0.65).14 Therefore, it is reasonable to consider use of DEB instead of DES in ISR, when available, reserving additional layers of DES in case of DEB failure.
Physiology and Image Guidance
Physiology-guided Percutaneous Coronary Intervention
The DEFINE REAL study added to available data on management of multivessel disease. This study included 484 patients with multivessel coronary artery disease of at least 40% stenosis by visual assessment on coronary angiography.15 Operators were asked to define their initial management plan based on diagnostic angiography.14 Subsequent invasive physiology was performed with either instant wave-free ratio (iFR) or fractional flow reserve (FFR) and a final management plan was developed. There was two- and three-vessel disease present in 73.3% and 26.7% of patients, respectively. Lesions investigated were intermediate with median percentage stenosis (60%), median FFR (0.84), and median iFR (0.92).15 Reclassification of overall management increased with the number of vessels investigated (one vessel: 37.3%; two vessels: 45.0%; three vessels: 66.7%; p=0.002) and incorporating iFR in the decision process was associated with investigation of more vessels (p=0.04) and higher reclassification (p=0.0001).15 This study demonstrates that coronary artery disease management changes dramatically when decisions are based on physiologic data added to anatomic data.
The 5-year outcomes of the Fractional flow reserve versus Angiography for Multivessel Evaluation (FAME-2) trial was published in 2018, demonstrating continued benefit of PCI compared with medical therapy in patients with FFR <0.8.16 The rate of the primary endpoint of death, MI or urgent revascularization was lower in the PCI group than in the medical therapy group (13.9% versus 27.0%; p<0.001).17 There were no significant differences between the PCI group and the medical therapy group in the rates of death or MI.16 Relief from angina was more pronounced after PCI compared with medical therapy.17 This trial supports the role of PCI for improving symptoms of angina and the need for urgent revascularization over medical therapy alone.
There are several physiologic measures that can be used to guide PCI. A meta-analysis of 23 studies involving 6,381 cases of stenosis comparing iFR to FFR was published in 2018 by De Rosa et al.18 They found the two indices were highly correlated (0.798 [0.78–0.82]; p<0.001). In a pooled per protocol population (n=4,486) of the Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularization (DEFINE-FLAIR) trial and Instantaneous Wave-Free Ratio Versus FFR in Patients With Stable Angina Pectoris or ACS (iFR-SWEDEHEART) trial, Escaned et al. evaluated the safety of deferral of coronary revascularization in FFR compared to iFR.19 Coronary revascularization was deferred in 2,130 patients and deferral was performed in 1,117 patients (50%) in the iFR group and 1,013 patients (45%) in the FFR group.19 Despite the higher rate of deferral in the iFR group, at 1 year the composite of all-cause death, non-fatal MI or unplanned revascularization in the deferred population was low (4%) and similar between the iFR and FFR groups.19 Additionally, if patients who presented with acute coronary syndrome were deferred, they had a significantly increased event rate when compared with those with stable angina at 1 year.19 These studies support the use of either measure for routine use in PCI.
Image-guided Percutaneous Coronary Intervention
The use of intracoronary imaging modalities was investigated in a large observational study involving 87,166 PCI patients from 2005 to 2015. Use of optical coherence tomography (OCT) compared with PCI with angiography alone, was associated with longer stent length. After propensity matching, mortality was lower in procedures with OCT (HR 0.39 [0.021–0.77]) but not different in OCT versus intravascular ultrasound (IVUS)-guided PCI (HR 0.88 [0.061–1.38]).16 Overall, this observational study supports the use of imaging, including IVUS or OCT, to guide PCI.
Devices
Drug-eluting Stents
In 2018, there were several reports of long-term outcomes of randomized clinical trials investigating the current generation of durable polymer DES. In the DUrable polymer-based sTent CHallenge of Promus ElemEnt versus ReSolute integrity (DUTCH PEERS TWENTE II) trial, which enrolled 1,811 all-comers to zotirolimus versus everolimus-eluting stents, 5-year incidence of target vessel failure (13.2% versus 14.2%) and definite or probable stent thrombosis were similar (1.5% versus 1.3%).20 The 5-year outcomes for the Clinical Trial to Assess the PROMUS Element Stent System for Treatment of De Novo Coronary Artery Lesions (PLATINUM), was also reported.21 This trial randomized 1,530 patients to the platinum-chromium everolimus-eluting stent (PtCr-EES) versus the cobalt-chromium everolimus-eluting stent (CoCr-EES) and demonstrated comparable safety and effectiveness with target lesion failure rates of 9.1% versus 9.3%, respectively (HR: 0.97; p=0.87). There were low rates of stent thrombosis and other adverse events.21 The 5-year outcomes were also acceptable in subgroup analysis for vessels that were <2.50 mm and lesions longer than 24 mm in length when treated with PtCr-EES.21 In this trial, the metal alloy did not influence PCI outcome.21 These results show excellent efficacy and safety of the current generation DES used in clinical practice and serve as a comparator for newer DES that are being developed.
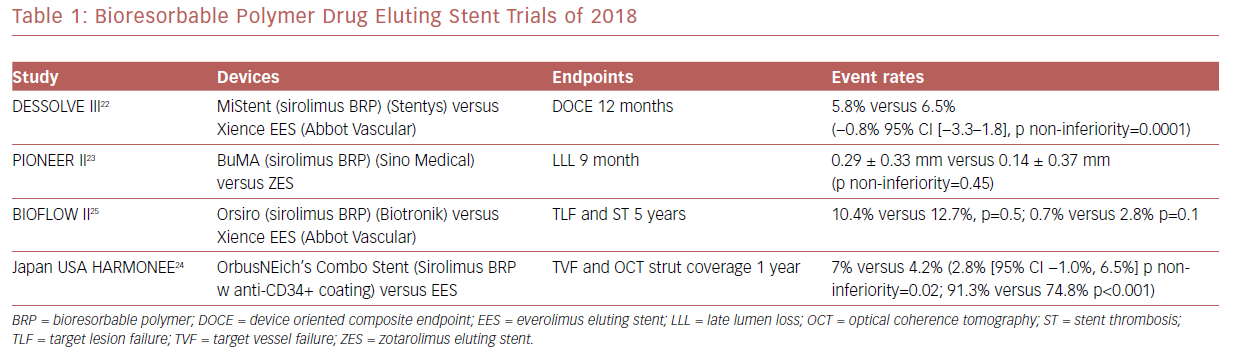
There have been several randomized clinical trials which showed promising results regarding bioresorbable polymer DES (Table 1). Among these was MiStent SES Versus the XIENCE EES Stent (DESSOLVE III), where the sirolimus-eluting bioabsorbable polymer stent (MiStent, Stentys) was compared with an everolimus-eluting durable polymer stent (EES) and found to be non-inferior at 12 months.22
A Study to Evaluate the Efficacy and Safety of BuMA Supreme Drug Eluting Stent (DES) (RCT) (PIONEER-II) was a non-inferiority trial that compared the BuMA Supreme bioresorbable polymer SES versus a contemporary durable polymer zotarolimus-eluting stent (ZES) in terms of angiographic in-stent late lumen loss at 9-month follow-up as the primary endpoint. At the 9-month follow-up, this primary endpoint was not met.23 PIONEER III, which is powered for clinical endpoints, is currently enrolling.
The Harmonized Assessment by Randomized Multicentre Study of OrbusNEich’s Combo StEnt (HARMONEE) study incorporated the use of OCT. This was a randomized trial comparing EES versus OrbusNeich’s Combo stent, which combined sirolimus and an abluminal BP with a novel endoluminal anti-CD34+ antibody coating designed to capture endothelial progenitor cells and promote healing. The combo stent did demonstrate non-inferiority for 1 year TVF in comparison with EES, with superior strut-based tissue coverage by OCT as a surrogate of EPC capture technology activity.24 Long-term, 5-year outcomes for the Study of the Orsiro Drug Eluting Stent System (BIOFLOW II) study demonstrated similar rates of target lesion failure and stent thrombosis in the Orsiro sirolimus BP stent compared with EES.25
Bioresorbable Vascular Scaffolds
The only bioresorbable coronary scaffold (BRS) approved in the US, the ABSORB everolimus-eluting scaffold (Abbot Vascular), was removed from the market in September 2017 for occurrence of very late scaffold thrombosis. Bioresorbable scaffolds continue to be under investigation because of their many promising features. Included among these trials is the randomized comparison of the NeoVas sirolimus-eluting BRS to a metallic EES in 560 patients. Eligible patients had a single de novo native coronary artery lesion with a reference vessel diameter of 2.5 to 3.75 mm and a lesion length ≤20 mm.26 Angiographic follow-up was performed in all patients at 1 year. The primary endpoint was angiographic in-segment late loss (LL), and the major secondary endpoint was the rate of angina. Baseline and follow-up OCT and FFR were performed in a pre-specified subgroup of patients. 1-year in-segment late loss with NeoVas and EES were 0.14 ± 0.36 mm versus 0.11 ± 0.34 mm.26 Additionally at 1 year, the two groups were similar in regards to the rates of recurrent angina.26 OCT at 1 year demonstrated a higher proportion of covered struts, less strut malapposition and a smaller minimal lumen area with NeoVas.26 Non-significant differences were found by FFR among the two groups.26 Longer-term outcomes are needed to determine the incidence of potential late scaffold issues. Future BRS platforms will have thinner struts and potentially more rapid absorption.
Conclusion
In 2018, there were advances across the spectrum of interventional cardiology, including the role of PCI in various clinical syndromes, techniques, and outcomes in a high-risk lesion subset, the design of stents, and use of functional and image-guided PCI. The future of PCI will continue to expand through research and bring advancements, including the increasing use of robotics, hemodynamic support, tele-stenting, and artificial intelligence, with the goal of improving and extending the lives of patients who have coronary artery disease.