Physiological lesion assessment is recommended for the identification of intermediate coronary lesions that might benefit from percutaneous coronary intervention (PCI).1 The quantitative flow ratio (QFR) was developed to derive coronary physiology from angiographic images, whereas the optical flow ratio (OFR) is a more recent approach for the rapid and automated assessment of coronary physiology from intracoronary optical coherence tomography (OCT).2–5 The aim of this article is to present an overview of the evidence, clinical applications, and future perspectives supporting the use of QFR and OFR for the physiologic assessment of intermediate coronary lesions in the catheterization laboratory.
Indication and Use of Physiologic Lesion Assessment in the Catheterization Laboratory
Visual estimation limitations of the severity and extent of epicardial coronary artery disease have been acknowledged for more than two decades.6,7 Fractional flow reserve (FFR) and instantaneous wave-free ratio (iFR) are indices that reveal the extent to which an epicardial coronary stenosis causes a pressure drop as a surrogate for compromised coronary flow.8,9 FFR and iFR assessment have a class 1A recommendation in the 2019 European Guidelines on Chronic Coronary Syndromes for high-risk patients without documented ischemia and for whom revascularization is considered.1 The use of wire-based physiologic assessment is improving, but is still underutilized because of a lack of confidence in visual assessment, prolonged procedure time, high cost, and risks related to pressure wires.10,11
Emerging Tools for Image-based Derivation of Fractional Flow Reserve
Multiple efforts were recently made to develop post-processing computational methods to derive FFR from imaging data.12–16 Invasive coronary angiography (ICA)-derived FFR solutions have the potential to expand the use of physiologic-guided PCI and reduce the amount of pressure wires needed. Intracoronary imaging-derived FFR solutions are able to integrate physiologic and morphologic information in a one-step approach. The current review focuses on the ICA-derived QFR and OCT-derived OFR.
Quantitative Flow Ratio
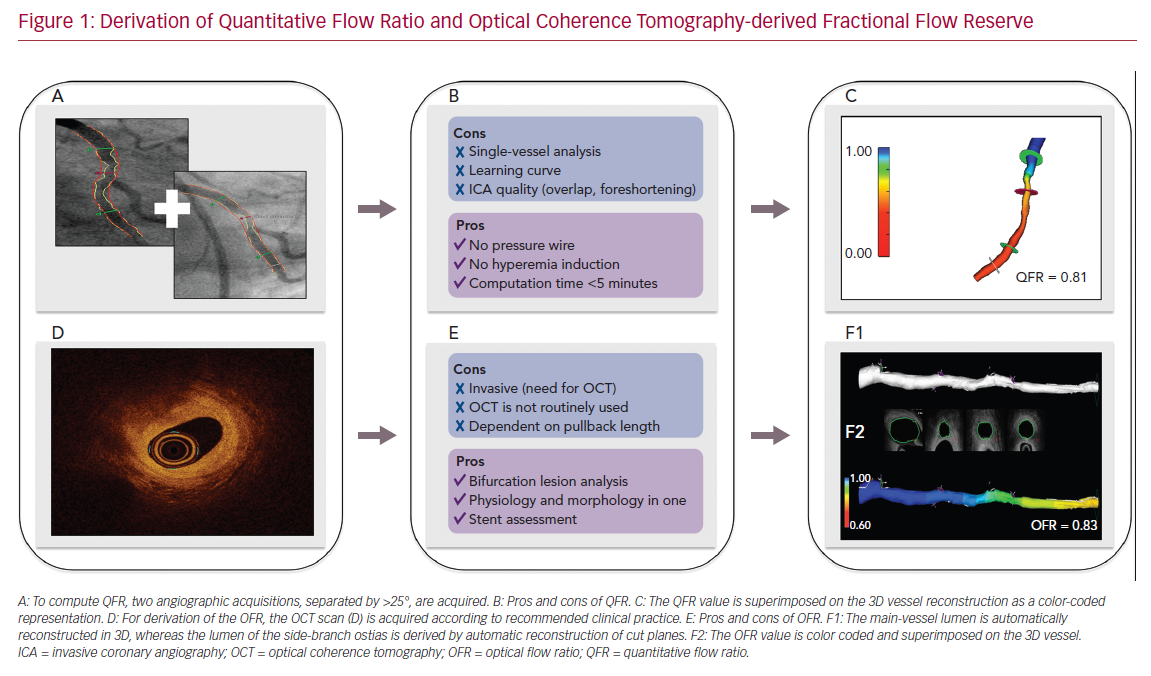
The backbone of FFR computation is based on accurate reconstruction of the coronary geometry and hemodynamic modeling. To reconstruct the coronary geometry, 3D quantitative coronary angiography (3D-QCA) is performed with the use of a minimum of two angiographic acquisitions, usually separated by ≥25° (Figure 1). For the calculation of pressure drop and flow reserve, initial ICA-derived FFR computation approaches were based on complex and time-consuming computational fluid dynamic solutions.17,18 To facilitate implementation in routine clinical workflow, QFR was developed. Empiric fluid dynamic equations were applied to shorten the computation time.2 Three different blood flow models are available for QFR. The first uses a fixed hyperemic flow velocity of 0.35 m/s (fQFR). The second uses contrast-flow velocity as derived by the thrombolysis in MI (TIMI) frame count from diagnostic coronary angiography to predict hyperemic flow velocity (cQFR). Finally, hyperemic flow velocity can be derived with TIMI frame counting during adenosine infusion. In clinical practice, the use of cQFR is recommended. Despite the need for manual interaction, the total cQFR in-procedure computation time is on average 5 minutes.19,20 QFR computation is commercially available from QAngio XA 3D software (Medis Medical Imaging Systems), as well as AngioPlus (Pulse Medical Imaging Technology), which uses the same QFR computational algorithm.
Optical Coherence Tomography-derived Fractional Flow Reserve
Intravascular imaging visualizes the coronary artery lumen with high resolution. Tools have improved substantially with regard to automated lumen and stent detection.21 Consequently, several groups have attempted to derive FFR-based geometric reconstructions from intracoronary imaging.16 OCT can visualize the vessel structure with a resolution much higher than that of ICA and resolve the limitation of vessel overlap and foreshortening by ICA. Therefore, a computational approach similar to QFR was applied to OCT images for the derivation of OFR.4 Following the acquisition of an OCT pullback according to routine clinical practice, the lumen contours are automatically delineated and stacked in 3D. The cut planes of the side-branches ostia are reconstructed to quantify the side-branch areas. The latter allows for an accurate derivation of the step-down reference lumen as if there were no stenosis (Figure 1). Finally, the volumetric flow rate is derived by multiplying a fixed-flow rate of 0.35 m/s with the reference lumen size, and OFR at every cross-section is computed and superimposed with the OCT images. OFR computation is commercially available from OctPlus software (Pulse Medical Imaging Technology). A recent study showed that the diagnostic performance of OFR was superior to QFR, likely due to the incorporation of side-branches and accurate OCT-derived lumen dimensions.5
Clinical Applications
Identification of Coronary Artery Stenosis with Indication for Revascularization
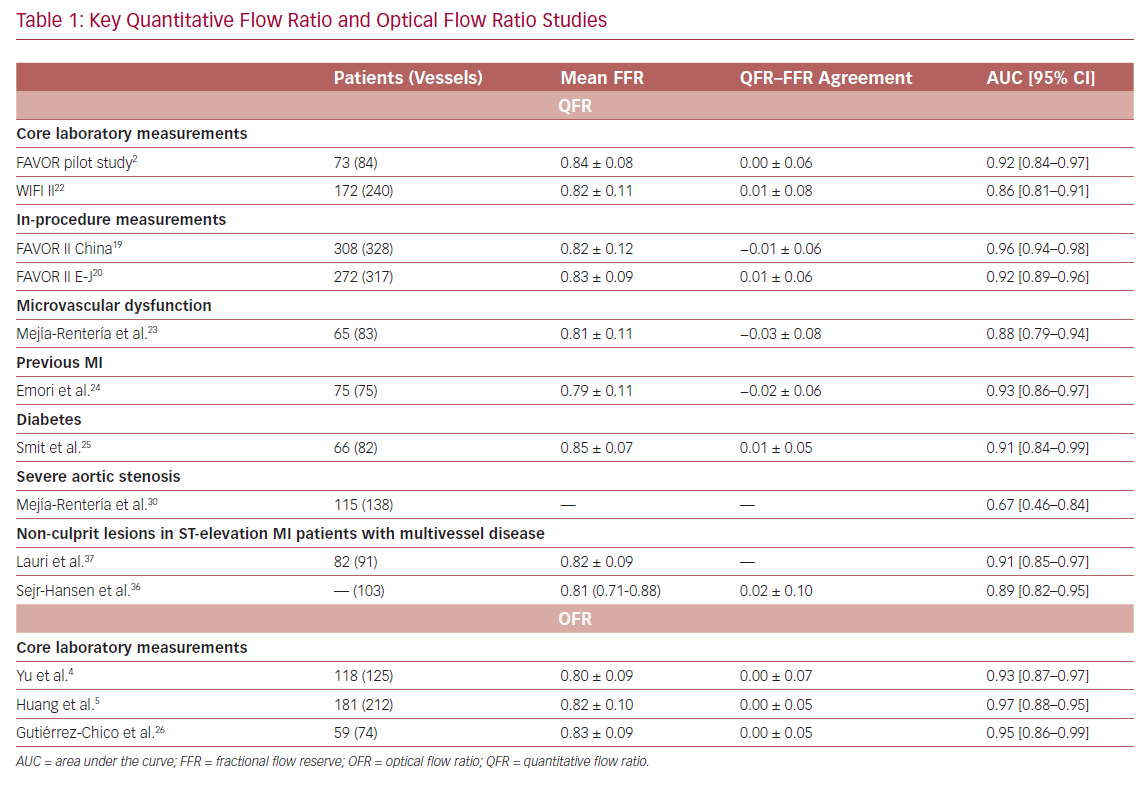
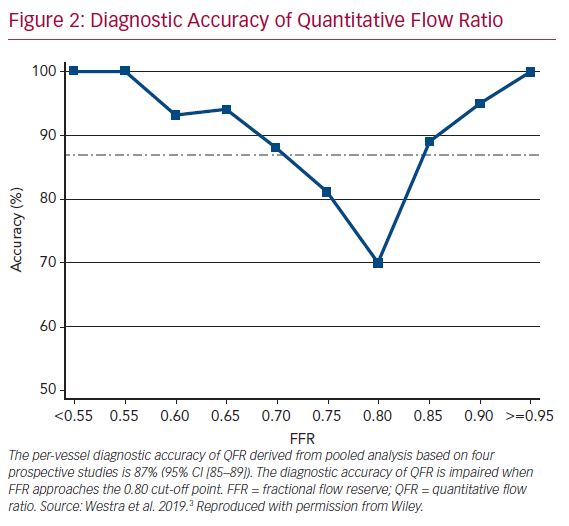
It is widely accepted that an FFR ≤0.80 is a good indicator for vessels to benefit from revascularization.1 Therefore, FFR was routinely used as a reference standard in diagnostic studies evaluating new physiologic indices. QFR and OFR have good diagnostic and numerical agreement with FFR in retrospective, prospective offline, and prospective in-procedure studies (Table 1).2,4,5,19,20,22–26 The Functional Assessment by Virtual Online Reconstruction (FAVOR) II trials compared the diagnostic performance of in-procedure QFR, with the FFR as a reference standard, and found high diagnostic accuracy. Importantly, QFR was measured within the time of conventional FFR measurements. The diagnostic certainty drops close to the FFR 0.80 cut-off point, where the benefit of revascularization is questionable (Figure 2).27,28 Observational data show more discordance between QFR–FFR in patients with chronic kidney disease, diabetes, previous MI, microcirculatory dysfunction, severe stenosis (high percentage diameter stenosis or long lesion length), and severe aortic stenosis (aortic valve area <0.60 m2).3,23,24,29,30 However, the overall validation results appear comparable and promising (Table 1). QFR was further compared to non-invasive imaging with heterogeneous results.31,32 An increased distal microvascular resistance and impaired ability to dilate the microvasculature could contribute to the described QFR–FFR discordance rate observed in patients with diabetes, previous MI, and microcirculatory dysfunction. However, it is unclear which index provides a ‘true’ measure of the epicardial lesion severity in the setting of increased microvascular resistance, because FFR is inherently affected by microvascular dysfunction.33
Physiologic Lesion Assessment in ST-elevation MI Patients with Multivessel Disease
Current guidelines recommend the use of functional testing to identify non-culprit lesions with an indication for revascularization.34 However, the validity of invasive physiology in the setting of ST-elevation MI (STEMI) remains a subject of debate.35 Transient changes in the downstream resistance and resting versus hyperemic flow could affect indices, such as FFR and iFR, differently depending on the time of measurement (acute/staged). The evaluation of non-culprit lesions with QFR in the acute setting of STEMI has been found to be comparable to the evaluation of the same lesions using QFR or FFR in a staged setting.36,37 The use of QFR in the setting of STEMI and multivessel could reduce the acute procedure length (e.g. QFR can be computed both in-procedure or post-hoc based on the acute angiography) and decrease the need for further downstream evaluation of non-culprit lesions.
Physiologic Lesion Assessment in Patients with Severe Aortic Stenosis
Patients with symptomatic severe aortic stenosis often have concomitant coronary artery disease warranting revascularization.38 The identification of flow-limiting stenosis in the setting of severe aortic stenosis could be challenging, because wire-based physiologic indices are susceptible to changes in coronary hemodynamics induced by aortic stenosis.39 However, despite that, the variations in FFR pre-transcatheter aortic valve replacement (TAVI) versus post-TAVI are potentially small, and the hemodynamic effects related to the administration of vasodilators in the setting of severe aortic stenosis might hamper its use.40 Therefore, QFR appears to be superior to quantitative coronary angiography for the identification of flow-limiting lesions in patients with coronary artery disease and aortic stenosis scheduled for TAVI.30
Optimizing Percutaneous Intervention
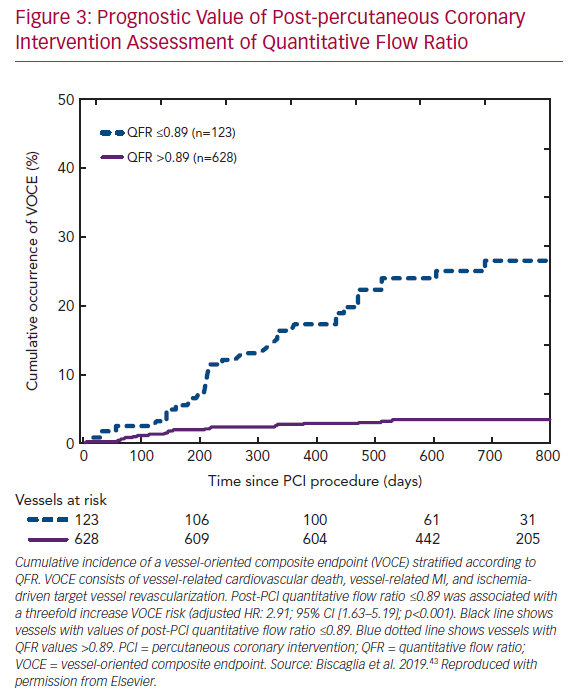
Recent data from the Physiologic Assessment of Coronary Stenosis Following PCI (DEFINE PCI) study illustrated that a residual pressure drop (as measured with iFR) is present in one in five cases with angiographic-successful PCI.41 Importantly, four of five cases had residual focal lesions with the potential for further optimization by PCI. Furthermore, multiple large studies confirmed that post-PCI FFR is a predictor of clinical outcome.42 Low post-PCI QFR after successful revascularization was found to predict a vessel-oriented composite endpoint consisting of vessel-related death, vessel-related MI, and ischemia-driven target vessel revascularization in the Angio-based Fractional Flow Reserve to Predict Adverse Events After Stent Implantation (HAWKEYE) study (Figure 3).43 Therefore, the routine use of post-PCI QFR might be a swift and straightforward solution for the identification of suboptimal PCI results, as the measurements can easily be acquired using two standard post-PCI angiographic acquisitions. Nevertheless, it should be noted that post-PCI QFR cannot completely assess the impact of stent malapposition in computational physiology, as coronary angiography fails to visualize in-stent conditions.
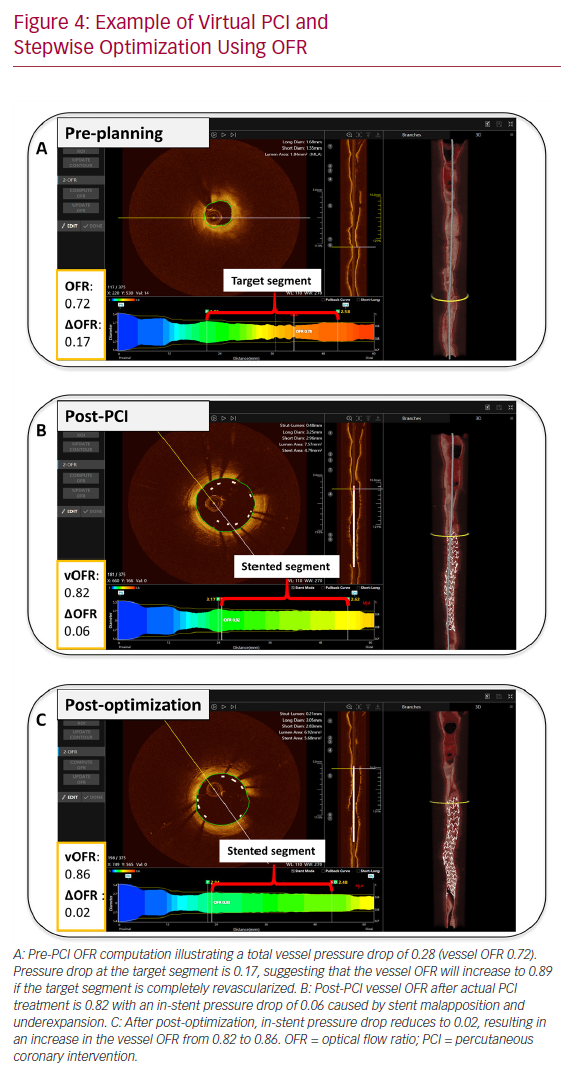
The role of intracoronary imaging for procedural guidance is increasingly acknowledged, given the ambiguity of angiography in several clinical settings, such as left main coronary artery disease and coronary artery disease involving bifurcations.44 In a large meta-analysis, the use of intravascular ultrasound or OCT was associated with a reduction in cardiovascular mortality when compared to ICA-guided PCI.45 Therefore, the structured, stepwise use of OCT with OFR as an add-on modality could provide instant evaluation of physiology, lumen dimensions, dissections, stents, and wires, potentially improving the final PCI results (Figure 4).
Virtual Percutaneous Intervention Planning
Similar to lumen dimensions measured by OCT, 3D-QCA provides the operator with detailed vessel dimensions, which are useful for treatment planning.46 Furthermore, QFR software enables the calculation of residual QFR, predicting the effect of stenting individual lesions that might be useful in cases with tandem lesions or diffuse disease. However, there are insufficient data on the current application. Initial pilot data from the Does Optical Coherence Tomography Optimize Results of Stenting (DOCTORS) study found a correlation between pre-PCI residual QFR (virtual PCI) and the actual post-PCI FFR results.47,48 Of note, such a correlation might be reduced by the presence of in-stent pressure drop as result of suboptimal PCI. That is, virtual PCI assumes that the target vessel segment is completely revascularized (Figure 4). Additionally, QFR and OFR provide the user with a pressure pullback, as known from FFR, that can be useful for differentiating focal disease from diffuse disease. The pullback pressure gradient was recently introduced to identify arteriosclerotic disease patterns with motorized FFR pullbacks.49 This might enable more individualized patient decision-making by identifying diffuse disease that can often be managed with optimal medical therapy or coronary artery bypass graft. Given the concordance of QFR and OFR with FFR, the virtual pullbacks derived from QFR and OFR might be similarly suitable for this purpose and will be subject to future study.
Identification of Microvascular Dysfunction
Epicardial coronary artery disease and coronary microvascular disease (MVD) can be difficult to differentiate, but can co-exist.50 Therefore, it is important to look for MVD, as it could explain persistent symptoms in a large proportion of symptomatic patients that undergo ICA without obvious disease or persistent symptoms after successful PCI in obstructive epicardial disease. Contrary to the fixed-flow QFR algorithm, the contrast-flow QFR incorporates the TIMI frame count to estimate the hyperemic flow velocity. It was therefore hypothesized that a large difference between contrast-flow QFR and fixed-flow QFR could occur in the setting of MVD. In a proof-of-concept study, a >0.07 difference between cQFR and fQFR was considered an independent predictor of MVD, as assessed with contrast-enhanced cardiovascular magnetic resonance after PCI.51 If future studies confirm these findings in various patient settings, an approach integrating all QFR algorithms might be applicable for differentiating epicardial and microvascular disease.
Perspectives
Toward Patient-tailored Treatment Strategies: Combination of Physiology and Morphology
Patients with recurrent acute coronary syndrome have a greater prevalence of high-risk plaque features, such as thin-cap fibroartheromas when compared to stable patients.52 Approximately half of all patients presenting with STEMI and multivessel disease have vulnerable plaque morphology, as detected by OCT in obstructive non-culprit lesions. The latter findings were presented in the COMPLETE-OCT substudy, and might explain the favorable clinical outcome found in the Complete vs Culprit-only Revascularization to Treat Multi-vessel Disease After Early PCI for STEMI (COMPLETE) study.53,54 In the COMPLETE study, a small proportion of patients underwent FFR-guided revascularization (<1%). Therefore, the question remains of whether physiology-guided complete revascularization has a benefit in the setting of STEMI and multivessel disease.55 OFR could play a future role by providing vessel morphology with the identification of high-risk plaque features, whereas the physiology component is able to identify flow-limiting lesions. This synergy could potentially enable the treating physician to provide better patient-tailored treatment.
Outcome Trials
It is unknown whether the concordance of QFR (and OFR) with FFR translates into comparable clinical outcomes. Three major randomized clinical outcome trials are currently ongoing. The FAVOR III (China) trial is testing the hypothesis that a QFR-guided PCI strategy results in a superior clinical outcome compared to a standard angiography-guided PCI strategy after 12 months (NCT03656848). The FAVOR III E-J trial is testing the hypothesis that a QFR-based diagnostic strategy results in a non-inferior clinical outcome when compared to a FFR guided strategy after 12 months (NCT03729739). The QFR Guided Revascularization Strategy for Patients Undergoing Primary Valve Surgery With Comorbid Coronary Artery Disease (FAVOR IV-QVAS) is testing the hypothesis that a QFR-guided strategy can reduce the incidence of a composite outcome within 30 days after surgery, as compared to an ICA-guided strategy in patients with planned primary valvular surgery and concomitant coronary artery disease (NCT03977129).
Conclusion
QFR and OFR have the potential to improve clinical practice by providing detailed coronary anatomy and imaging derived physiology. The integration of anatomy and physiology could inform clinical decision-making for treatment and optimize subsequent revascularization if indicated. Validation of the techniques through ongoing randomized outcome trials are needed to prove the efficacy of these solutions when applied by users outside highly trained core laboratories.