More than 10 million Americans suffer annually from angina.1 For decades, most of the attention has been focused on epicardial coronary artery disease (CAD). In a European registry of 11,000 stable angina patients, 65 % of women and 32 % of men had no obstructive CAD (<50 % stenosis); however, multiple other studies have demonstrated only 30 % of patients have significant obstructive epicardial CAD.2 In 1973 Harvey Kemp was one of the first to describe ‘cardiac syndrome X’ in patients with typical angina pectoris with ischemic electrocardiographic changes but in the absence of epicardial obstructive CAD.3 The terms ‘cardiac syndrome X’, ‘microvascular angina,’ or ‘chest pain with normal coronary arteries’ have been used interchangeably in the literature referring to the chest pain associated with coronary microvascular dysfunction (CMVD). There is some impetus to abandon the term syndrome X given the lack of a standard definition. Patients with CMVD have higher endothelium-dependent and endothelium-independent impairment of microvascular function.4 Coronary flow reserve (CFR), which is the ratio of coronary blood flow (CBF) at maximal dilatation to CBF at rest, is abnormal in patients with microvascular dysfunction. Hypertension, insulin resistance, and hyperlipidemia are well-known risk factors.5 While women have been consistently found to have less obstructive CAD than men, many reports have demonstrated a higher prevalence of CMVD in women, including one study in which 55 % were women.6 In the Women’s Ischemia Syndrome Evaluation (WISE) trial, angina was linked to increased mortality at 5 years.7 In fact, in patients with angiographically documented non-obstructive disease, the risks are the same as patients with significant single-vessel disease.7 During the last decade CMVD has been validated as a significant cause of myocardial ischemia and has also been associated with a wide range of diseases. Approximately 20–30 % of patients with successful coronary bypass surgery will continue to suffer from angina, which may result from microvascular disease.8 CMVD may be responsible for ‘false positive’ stress tests in which ischemia is detected but without significant or even ‘normal’ coronaries visualized by coronary angiography.
More recently CMVD has been linked to the development of heart failure with preserved ejection fraction (HFpEF)9 and Takotsubo syndrome.10,11 Patients with various chronic inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosus, also have an increased risk of premature CAD. Risk factors inducing oxidative stress and inflammation may trigger endothelial dysfunction, and thus altered vasomotion of the microcirculation.12
Given the increasingly recognized importance of microvascular disease, we present this review focusing on the mechanisms leading to CMVD, its diagnostic evaluation, as well as possible treatment options, and propose a practical diagnostic algorithm for CMVD.
Microvascular Dysfunction Pathophysiology
Several theories are suggested to explain the pathophysiology of CMVD. This involves both pre-arterioles (vessels of 100–500 µm in diameter) and arterioles (<100 um), which are the resistance vessels responsible for the abnormal coronary artery blood flow. Structural alterations, including smooth muscle hypertrophy around the vascular wall, results in increased vessel resistance and is seen in hypertrophy from left ventricular hypertrophy secondary to hypertension or hypertrophic cardiomyopathy, and have been described as part of CMVD mechanism;13 however, other studies have failed to establish any mechanism.14 Patients with CMVD have similar risk factors as those with epicardial CAD. CMVD can occur in patients with and without obstructive CAD.
Functional abnormalities are likely the most widely accepted and recognized mechanism of CMVD. Dysfunction of microcirculation resistance has been reported in a large number of studies. An impairment of endothelium-dependent vasodilation due to reduced nitric oxide (NO) release is among the most commonly proposed mechanisms of CMVD in stable microvascular angina (MVA) patients.
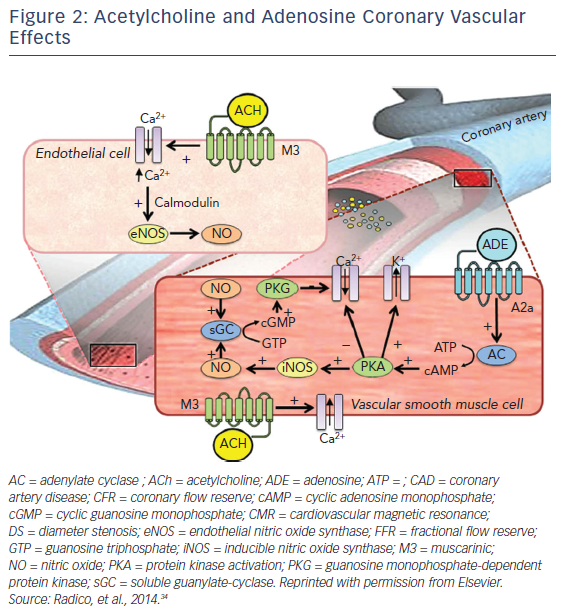
In addition to NO, acetylcholine (ACh) governs the endothelial-independent mechanism, which has been proven by reduced CBF to ACh. Both, endothelium-dependent and endothelium-independent need to be tested by means of coronary reactivity response to adenosine and ACh. ACh has dual antithetical effects on the coronary arteries by binding to muscarinic (M3) receptor on the surface of vascular smooth muscle cells and elicits an intracellular release of calcium ions leading to vasoconstriction, whereas endothelial M3 receptor-mediated calcium release activates the endothelial NO synthase (eNOS or NOS3) through a calmodulin-dependent pathway. NO is then released and, in vascular smooth muscle cells, activates soluble guanylate-cyclase, which converts guanosine triphosphate into cyclic guanosine monophosphate. The subsequent activation of guanosine monophosphate-dependent protein kinase induces a cascade of intracellular events with the final effect of decreasing intracellular calcium concentrations, leading to vasodilation. Adenosine binds to its receptors (A2a) on the surface of vascular smooth muscle cells, activating adenylate cyclase and leading to an increase in cyclic adenosine monophosphate (cAMP) concentration and cAMP-dependent protein kinase activation. The latter results in potassium channel opening, resulting in a hyperpolarization of vascular smooth muscle cells, inhibits the entry of calcium and also activates inducible NOS (iNOS or NOS2), thus producing vasodilation.15–17
While there is a reduction in the endothelium-dependent vasodilation due to changes in CBF, this does not seem sufficient to fully account for CMVD in these patients. The potential direct vasoconstrictor effects of ACh are not highly specific for the assessment of endothelial function, and in some studies, the metabolic pathway of NO did not seem to be affected.18 Other studies have shown enhanced vasoconstrictor activity in coronary microcirculation in several patients with stable MVA.
Ergonovine injection, mental stress, and hyperventilation can all result in an impairment of CBF. In some patients, ACh-induced angina was associated with a reduction of CBF in the absence of epicardial vasoconstriction, and even exercise was suggested to induce vasoconstriction rather than vasodilation. Of note, endothelin-1 serum levels were found to increase in the coronary sinus during atrial pacing. Finally, in some typical patients with cardiac syndrome X, basal microvascular constriction was suggested by the evidence of slow coronary flow.
Thus, several structural and functional alterations have been described in patients with stable MVA. The reduced CBF response to direct arteriolar vasodilators (dipyridamole, adenosine) with induction of ischemic ST-segment changes and angina, however, suggests a major role for a primary increased vasoconstriction of small coronary resistive vessels. Indeed, the poor response to vasodilators of these areas can allow a blood steal phenomenon by normal microvessels, resulting in microvascular ischemia. Accordingly, microvascular constriction may limit the vasodilator response to exercise (thus favoring effort angina) and in some cases may also facilitate the impairment of myocardial blood flow, and thus angina at rest. It has been difficult to obtain clear evidence of myocardial ischemia in patients with stable MVA. In addition, the patchy distribution of CMVD, which in turn may not lead to wall motion abnormalities or poor evidence for abnormal perfusion, can be due to several other reasons, including: the inappropriateness (type and/or dose) of the stress stimulus; the limitations of current technical methods to detect minor degrees of myocardial ischemia; and the intermittent nature of CMVD.
Testing in Microvascular Angina
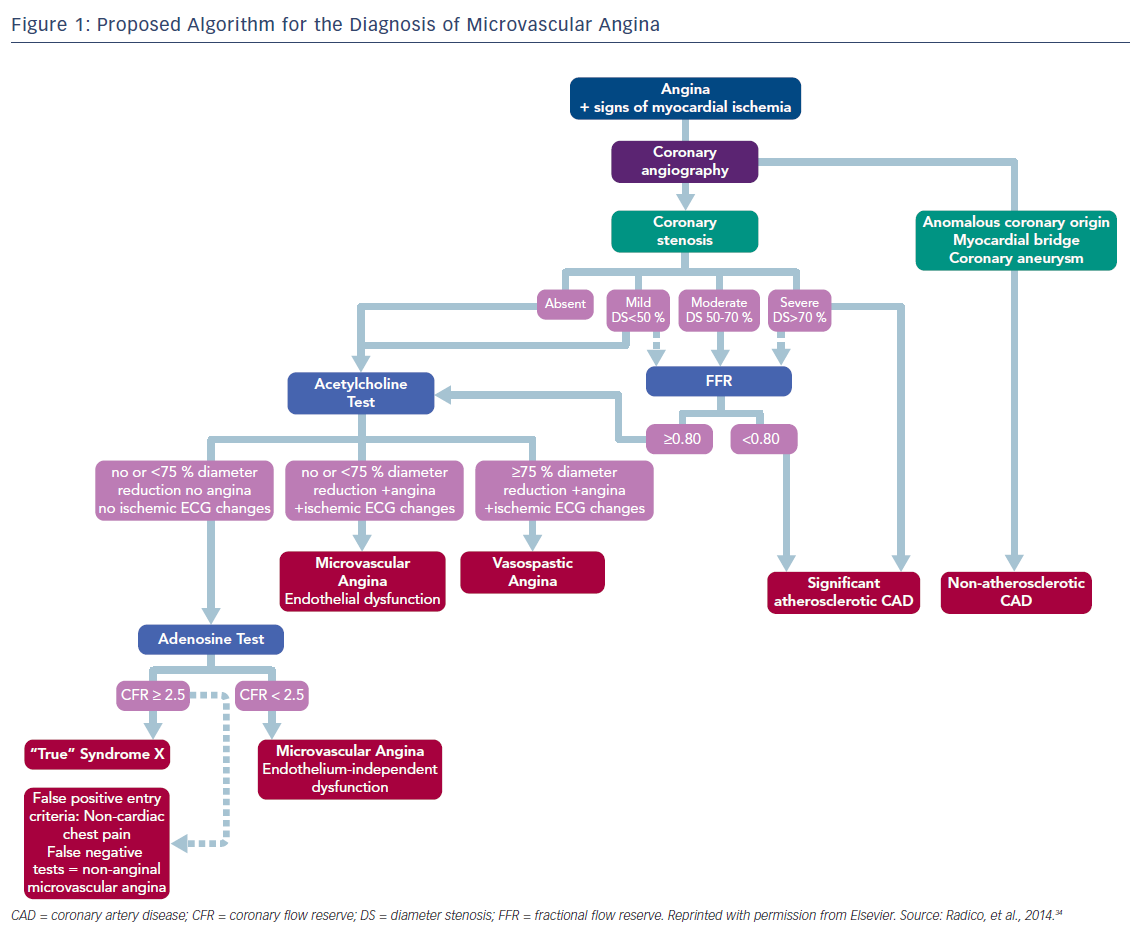
Multiple diagnostic modalities have been proposed to diagnose patients with microvascular dysfunction. Both invasive (thrombolysis in myocardial infarction [TIMI] frame rate, intracoronary Doppler flow wire [IDFW] recording with coronary reactivity testing) and noninvasive (PET, CT, cardiac magnetic resonance imaging) tests can be used.19,20 Evidence for ischemia with non-obstructive CAD by ECG, decreased tissue perfusion detected by an increased TIMI frame count that measures myocardial blush grade, or transient perfusion defects on adenosine stress imaging may warrant further evaluation for microvascular disease.
The microvascular function can be evaluated indirectly by determining the CFR, which is measured by using vasoactive agents such as adenosine, dipyridamole (endothelium-independent vasodilator), or ACh (endothelium-dependent).19,20
Noninvasive Testing in Microvascular Angina
Transthoracic Echocardiographic Doppler Echocardiogram
With varying degrees of difficulty, transthoracic echocardiographic Doppler echocardiogram (TEDE) can be an initial diagnostic test to evaluate the microvascular function. TEDE can sometimes visualize the left anterior descending, and by using color Doppler flow mapping estimate CBF by calculating the ratio between the diastolic peak flow velocity during the maximal vasodilation (e.g. adenosine) and the coronary flow velocity at rest. A ratio less than 2.0 (normal 2.5–5.0) is used as a marker of microvascular dysfunction, and it correlates with invasive testing in the 85–97 % range. Finally, TEDE is inexpensive, reliable, and widely available.20–22
Cardiac Magnetic Resonance Imaging
CMR is one of the most reliable tests for evaluation of microvascular dysfunction. CMR can also assess the cardiac anatomy and general cardiovascular function. A vasodilator agent such as adenosine is used to increase the CBF, and gadolinium is used to enhance visualization of the sub-endocardium region. Changes in gadolinium enhancement are correlated with an abnormal coronary flow and microvascular dysfunction. Unfortunately, CMR is expensive, can be limited by motion artifact, and challenging in patients with claustrophobia and implanted devices.19–21,23
Positron Emission Tomography
PET is considered one of the most reliable methods to evaluate microvascular dysfunction. PET uses a flow tracer that allows the determination of total regional myocardial blood flow at rest and after a vasodilator challenge. Unfortunately, PET is expensive, not broadly available, and its use in the daily clinical practice is very limited to specialized centers.21
Computerized Tomography
CT is one of the newest noninvasive methods to diagnose microvascular dysfunction. After injection of contrast, computational fluid dynamics are obtained in a specific anatomical segment. Coronary flow and pressures are calculated using mathematical models, and thus allow evaluation of the microvascular function.
New multidetector CTs have an excellent spatial resolution, cost-effectiveness, and allow the evaluation of the entire heart, albeit at the expense of radiation exposure.21,22,24
Invasive Methods
Several invasive techniques have been used to evaluate microvascular function, including thermodilution, gas washout method, and intracoronary Doppler flow wire. By far, flow wire assessment is the most common technique used.21
Intracoronary Doppler Flow Wire
IDFW is considered the gold standard in the evaluation of coronary microcirculation. IDFW directly measures the CBF velocity, direction, and pressure in an epicardial artery. Additionally, IDFW can evaluate the response to intracoronary injection of vasodilators and vasoconstrictors medications. A ratio of resting versus maximal hyperemia post adenosine infusion will allow for a cutoff between abnormal and normal microvascular function.21,24
Treatment in Microvascular Angina
Currently, the evidence for effective therapy in the treatment of CMVD is limited as there are no large randomized trials available. Therefore, most clinicians will treat CMVD with traditional antianginal therapies that have not necessarily been shown to improve patient outcomes. Studies usually lack specificity and involve patients with cardiac chest pain that may be attributed to other etiologies. Furthermore, the studies have limited contributions because of their small sample sizes, short-term follow-up periods and a lack of a universally agreed upon definition for CMVD. CMVD has been classified as occurring with normal coronary arteries, obstructive CAD, and with underlying structural myocardial disease. Therefore, treatment may also be variable depending on the type of CMVD. Most importantly, no studies have assessed whether treating CMVD will result in any long-term prognostic benefits.25
Given that the prognosis of patients with non-obstructive CAD is the same as single vessel disease, it is important that these patients be monitored routinely, managed aggressively, and not ignored and labeled with a diagnosis of non-cardiac chest pain.26 Lifestyle modifications remain of utmost importance. Patients should specifically be encouraged to exercise, participate in weight loss programs, and stop smoking.26,27 In addition to these modifiable risk factors, diabetes and hypertension should be strictly controlled and focus on similar parameters to those used in CAD.27
Most of the data collected for the medical management of CMVD have been extrapolated from several meta-analyses all utilizing a similar definition for CMVD. The diagnosis was defined as CFR <2.5 using PET, CMR, invasive coronary Doppler, or invasive intracoronary thermodilution. Patients with evidence of epicardial CAD (>50 %) were excluded.25
The studies that evaluated sildenafil, quinapril, enalapril, atenolol, estrogens, and TENS application demonstrated benefits in their respective endpoints.28 It should be noted that studies of long-acting nitrates have shown no positive effect on microvessels as they do in the epicardial vessels, and thus are not recommended.29
In a study, sildenafil showed improvement in CFR but was not assessed for symptomatic relief. The WISE control trial demonstrated improvement in angina and CFR with the use of quinapril.30 Atenolol was shown to reduce the number of angina symptoms.30 Other medications such as ranolazine, ivabradine, fasudil, and nicorandil continue to be considered and studied in the treatment of CMVD.27
Preliminary data suggests endothelin-1 antagonists or rho-kinase inhibitors may be useful in the prevention of endothelial cell dysfunction, vascular smooth muscle cell spasm, as well as the accumulation of inflammatory cells in the adventitia of vessels. Hence, by enhancing vaso-relaxation and reducing vascular inflammation both CFR and angina symptoms would likely show improvement. Finally, with the recent findings of the Cardiovascular Risk Reduction Study (Reduction in Recurrent Major CV Disease Events [CANTOS trial]) it would be reasonable to consider anti-inflammatory medications for future studies in the treatment of CMVD.31
However, it should be noted that some of the medications that showed benefit did not demonstrate this as a class effect. For example, fluvastatin alone showed improvement in CFR, exercise tolerance, and symptoms but pravastatin did not.32 In a similar fashion, verapamil and nifedipine have been shown to improve symptoms, require less nitrate usage, and improved exercise tolerance but were associated with fatal arrhythmias. Unlike verapamil, diltiazem did not show any benefit at all.33 Until further studies are completed truly satisfactory treatment options for this problem remains elusive.
Conclusion
While greater awareness of microvascular disease has occurred, all too often patients are dismissed as non-cardiac given the lack of epicardial disease. While women predominate it is a problem that occurs in men as well. Moreover, evidence now shows that the prognosis is not benign. Either noninvasive or invasive coronary reactivity testing should be undertaken. Risk factor modification and perhaps eventually anti-inflammatory treatment as well as anti-ischemic agents should then be initiated.