Heart disease remains the leading cause of mortality in the US, with acute MI from acute coronary syndrome (ACS) being a major contributor.1 Chest pain is one of the most common complaints in patients presenting to the emergency room (ER) in the US, with approximately 8–10 million annual visits.2 Multimodality cardiac imaging (e.g. cardiac CT, cardiac MRI) is playing an increasingly recognized role in the identification of at-risk patients, the planning of interventions, and in the understanding of the non-coronary causes of chest pain.
Advances in CT technology have improved spatial and temporal resolution, resulting in improved diagnostic capabilities. In the UK, coronary CT angiography (CCTA) has been recommended as a first-line imaging modality for patients with suspected coronary artery disease (CAD) since 2016.1 Similarly, the European Society of Cardiology (ESC) guidelines have endorsed the use of CCTA as a first-line imaging modality in the evaluation of chest pain since 2019.3 In the US, CCTA had not seen the same rate of adoption in the guidelines until the recently published 2021 multi-society guidelines for the evaluation and diagnosis of chest pain.4 CCTA now has a class I indication for the evaluation of chest pain in various scenarios, including intermediate-risk patients with acute chest pain without known CAD and intermediate or high-risk patients with stable chest pain and no known CAD.4 The 2014 American Heart Association/American College of Cardiology (AHA/ACC) guidelines for non-ST-elevation MI (NSTEMI) have assigned a class IIa recommendation for the use of CCTA in evaluating patients with possible ACS, with the caveats that a normal ECG, normal cardiac troponin, and no history of CAD be present.5
The challenge of imaging coronary arteries non-invasively is related to coronary anatomy and motion. Typically, being only millimeters in diameter and subject to motion artifact during the cardiac cycle, the achievement of the appropriate spatial and temporal resolution in the imaging of coronary arteries can be challenging. With the advent and more widespread availability of multi-slice multi-detector CT systems and other technological advancements, these hurdles have largely been overcome. Issues with image quality, however, can still exist. Specifically, noise, vascular enhancement, and coronary motion must all be optimized to achieve the best quality images.6 In this review, we highlight the role of CCTA in the evaluation of patients with acute chest pain and possible or confirmed ACS.
Role of CCTA in the Evaluation of Stable Chest Pain
Early studies focusing on the diagnostic performance of CCTA sought to evaluate the ability of this modality to detect significant coronary stenosis, usually defined as stenosis >50% on invasive coronary angiography (ICA). A 2018 meta-analysis compared the performance of several non-invasive diagnostic modalities to rule out significant CAD in patients with angina with either ICA or invasive fractional flow reserve (FFR) as the gold standards.3 When considering CCTA, a total of nine studies with ICA as the gold standard for comparison and seven studies with invasive FFR as the gold standard for comparison were included. When the ability of these non-invasive modalities to rule out anatomically or functionally significant CAD is compared, CCTA emerged as the best modality with a pooled sensitivity of 97% (95% CI [93–99%]) in detecting anatomically significant CAD and a pooled sensitivity of 93% (95% CI [89–96%]) in detecting functionally significant CAD, albeit at the expense of poor specificity of 78% (95% CI [67–86%]) and 53% (95% CI [37–68%]), respectively.
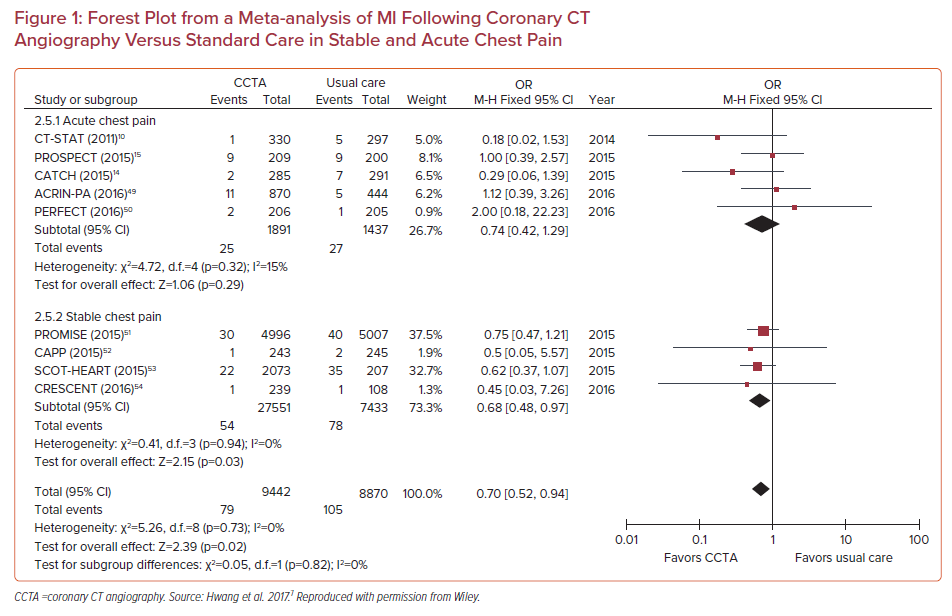
In a meta-analysis of 13 randomized controlled trials involving 20,092 patients (10,315 were assigned to CCTA, 9,777 assigned to functional testing), CCTA was associated with higher rates of CAD diagnosis (RR 2.80; 95% CI [2.03–3.87]), an increase in the prescription of both aspirin (RR 2.21; 95% CI [1.20–4.04]) and statins (RR 2.03; 95% CI [1.09–3.76]), and a reduction in MIs (RR 0.71; 95% CI [0.53–0.96]), but at the expense of increases in both ICA (RR 1.33; 95% CI [1.12–1.59]) and revascularization (RR 1.86; 95% CI [1.43–2.43]). This was also replicated in another meta-analysis of randomized trials comparing CCTA with usual care in stable CAD and acute chest pain (Figure 1).7
Taken together, CCTA is a powerful modality for ruling out significant CAD as the etiology of chest pain; it has superior sensitivity, albeit with worse specificity. The identification of early-stage plaques and higher rates of CAD diagnosis enables higher rates of initiation and intensification of preventive therapies, lowering the risk of MI. CCTA can act as a gatekeeper for unnecessary invasive catheterizations, where traditionally one-third of patients undergoing ICA have non-obstructive disease.8
CCTA in the Evaluation of Acute Chest Pain in the Emergency Room
Chest pain is among the most common causes of ER visits, with approximately 8–10 million patients evaluated for chest pain annually in the US alone.2 Compared with stable chest pain, patients with acute chest pain in the ER are at higher risk for adverse outcomes. A sensitive test for the evaluation of these patients is thus required to avoid unnecessary hospitalizations, reduce the length of stay (LOS) and cost, and provide a safe mechanism for ruling out significant CAD that may benefit from invasive therapies. Several trials have evaluated CCTA in the evaluation of chest pain in the ER in comparison with functional tests (Supplementary Material Table 1).9–18
The ROMICAT-II trial in 2012 randomized 1,000 patients presenting with acute chest pain (without ECG changes or troponin elevation) to early CCTA or standard evaluation in the ER.12 There was no difference between the study arms with respect to clinical outcomes, but CCTA resulted in more patients discharged from the ER (47% versus 12%, p<0.001), LOS reduced by 7.6 hours, and similar cost of care, albeit at the expense of increased downstream testing and higher radiation dose.12 The ACRIN-PA study randomized 1,370 patients with chest pain in the ER with TIMI scores 0–2 and no ischemic changes on ECG to CCTA or functional stress testing prior to evaluation of serum troponin. Only 84% of those randomized to CCTA underwent the scan and of those, 83% had no obstructive CAD. As has been demonstrated in stable chest pain populations, patients undergoing CCTA were more likely to be diagnosed with CAD (9.0% versus 3.5%), and less likely to have ICA without significant CAD. The 1-year event rate was very small and not different between the arms. That study provides practical evidence of the short-term safety of CCTA in low-risk patients and highlights its benefits.
The 2015 CATCH trial investigated the long-term clinical safety of CCTA in patients with recent-onset acute chest pain in the low- to intermediate-risk groups.14 High-risk patients were excluded based on either abnormal ECG, elevation in troponin, or recurrence of chest pain during a 24-hour hospitalization.14 A total of 600 patients were randomized to either CCTA or standard care (exercise ECG or single-photon emission CT) following discharge. The safety of CCTA in comparison with the standard care was established by assessing the composite of cardiac death, MI, hospitalization for unstable angina, late symptom-driven revascularization, and readmission for chest pain. The primary endpoint occurred significantly less frequently in the CCTA group than in the standard care group (HR 0.62; 95% CI [0.40–0.98]). The CCTA group was more likely to undergo revascularization compared with standard care, suggesting that the improved safety seen with the CCTA strategy may be related to timely revascularization.
In a systematic review of randomized controlled trials of CCTA (n=1,869 patients) versus usual care (n=1,397 patients) in the ER, there were no differences in outcomes (MI, death, ER visits, rehospitalizations). CCTA was associated with reduced LOS and ER costs, but it also led to an increase in ICA (OR 1.36; 95% CI [1.03–1.80]; p=0.030) and in revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG; OR 1.81; 95% CI [1.20–2.72]; p=0.004).19 In a meta-analysis of studies up to 2016 evaluating diagnostic accuracy in the ER without troponin elevation, coronary CT had the highest accuracy (sensitivity 93%; specificity 90%), which was comparable to myocardial perfusion scintigraphy but more accurate than stress ECG and echocardiography.20 A Markov microsimulation model comparing cost-effectiveness strategies in acute chest pain showed that although CCTA seems to increase short-term cost, CCTA was better than standard care and that recommended by the AHA/ACC guidelines. It also expedited ER discharge and the outpatient evaluation protocol mainly due to lower cardiovascular mortality.21 Supplementary Material Table 1 lists the major trials comparing CCTA with standard care in patients presenting to the ER with chest pain.
CT-derived FFR enables the evaluation of the hemodynamic significance of coronary stenoses, especially those in the intermediate range (50–70%) and renders many lesions non-hemodynamically significant, thus enabling timely safe discharge from the ER. The use of CT-derived FFR (FFRCT) in patients with acute chest pain was evaluated in two studies. In a substudy of the ROMICAT II trial, those with ≥50% stenosis in at least one coronary artery or those who underwent another non-invasive test were included, for a total of 116 patients.22 Due to technical limitations, such as motion and blooming artifacts, 68 patients successfully underwent FFRCT. An abnormal FFRCT (≤0.80) was significantly associated with the diagnosis of ACS compared with a normal FFRCT (57.5% versus 14.3%, p<0.001) and more often required revascularization (37.5% versus 10.7%, respectively). Furthermore, abnormal FFRCT was associated with high-risk plaque features as assessed on CCTA. In a real-world study of 555 low-risk patients (negative ECG and biomarkers) with chest pain presenting to the ER who underwent FFRCT (297CTA+ FFRCT and 258 CCTA only), the rejection rate for FFRCT was low (<2%), and there were no between-group differences with respect to clinical outcomes, diagnostic discordance with ICA, or cost. Negative FFR was more likely to be associated with non-obstructive disease (57% versus 8%).23 Future prospective studies comparing the diagnostic accuracy of FFRCT with CCTA involving confirmed NSTEMI patients at higher risk are under way, with the hopes of demonstrating a reduction in the need for ICA, procedure-related risks, and medical costs.24
The accuracy of CCTA in the evaluation of CAD in the ER has led to the development of protocols to simultaneously evaluate major causes of chest pain in patients presenting to the ER (CAD, pulmonary embolism, aortic dissection) through a ‘triple rule-out’ strategy. In a larger retrospective review of 12,834 patients who underwent CCTA of the chest (triple rule out, n=1,555; CCTA, n=11,279), the diagnostic yield for CAD was similar between the triple rule-out protocol and CCTA, with higher diagnostic yield for pulmonary embolism (1.1% versus 0.4%, p=0.004) and aortic dissections (1.7% versus 1.1%, p=0.046), at the expense of higher radiation (median 9.1 versus 6.2 mSv; p<0.0001), contrast load (mean 113 ± 6 ml versus 89 ± 17 ml; p<0.0001), and higher rates of non-diagnostic imaging (9.4% versus 6.5%, p<0.0001).25
Taken together, these data suggest that CCTA is an excellent tool for ruling out significant CAD in patients presenting to the ER with chest pain, with equivalent outcomes, possibly reduced cost and length of stay, at the expense of a small increase in ICA and revascularization. Table 1 lists the appropriateness of CCTA according to various guidelines and clinical scenarios.26
CCTA in NSTEMI
Clinical practice guidelines favor early invasive angiography for high-risk patients with elevated troponins/NSTEMI and selective ICA in patients based on clinical risk scores. However, up to 25% of patients with presumed NSTEMI do not have obstructive disease on ICA (this is known as MI with non-obstructive coronary arteries).27 This percentage may become even higher in the era of high-sensitivity troponin, with an estimated 50% of patients with a mild increase in high-sensitivity troponins having a normal ICA. Given the evolving role of CCTA in the evaluation of coronary atherosclerosis, it is important to discuss its emerging role in presumed NSTEMI. This role is highlighted by the 2020 ESC guidelines for the management of ACS without persistent ST-segment elevation.28 Specifically, the high negative predictive value (NPV) of CCTA for CAD allows for the exclusion of ACS.28 This has been demonstrated by several trials evaluating the diagnostic utility of CCTA in confirmed or presumed NSTEMI.
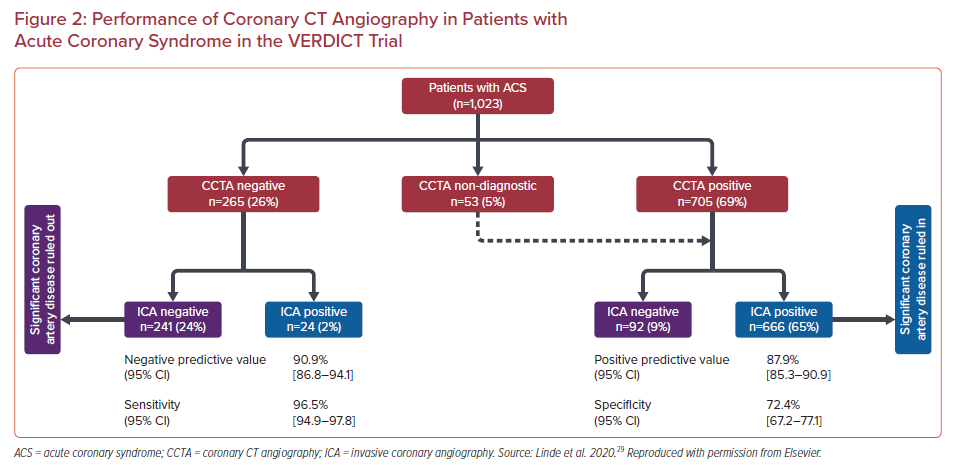
The accuracy of CCTA in the diagnosis of coronary stenosis in patients with non-ST-elevation acute coronary syndrome (NSTEACS) was recently evaluated in 1,023 patients with NSTEACS who underwent CCTA as well as ICA in the VERDICT trial (Figure 2).29 Significant (≥50%) coronary stenosis was diagnosed in 69% by CCTA and 67% by ICA. The per patient NPV of CCTA was 90.9% (95% CI [86.8–94.1%]) and the positive predictive value (PPV), sensitivity and specificity were 87.9% (95% CI [85.3–90.1%]), 96.5% (95% CI [94.9–97.8%]) and 72.4% (95% CI [67.2–77.1%]), respectively.29 This performance is similar to that of CCTA in stable disease (reported sensitivity of 97% and specificity 78%).3 The higher-risk population studied in the VERDICT trial in comparison with past studies of the diagnostic performance of CCTA did not affect the NPV. In fact, when only patients with a Global Registry of Acute Coronary Events (GRACE) score >140 are considered, the NPV of CCTA remained >95%, despite the high prevalence of 74% for significant CAD in this subgroup. An important caveat not captured by the numerical value of the PPV alone is that a threshold of ≥50% stenosis was used by the authors to define a positive test. Degree of stenosis alone does not completely predict the hemodynamic significance of a coronary lesion; and other factors, largely obtained via invasive procedures, must be considered before a decision about revascularization is made. In a high-risk population with a high incidence of coronary stenosis ≥50%, by using CCTA prior to ICA we may be subjecting most patients to unnecessary radiation and contrast. The population of the VERDICT trial is a good illustration of this given that 67% of them had at least one stenosis that occupied ≥50% of the vessel lms.
The RAPID-CTCA (NCT02284191) study randomized 1,748 patients in the UK with suspected ACS and ≥1 high-risk feature (prior CAD, elevated troponin >99th percentile, or ischemic changes on ECG) to early CCTA versus standard care and followed them for a 1-year rate of all-cause death or MI. There was no difference in clinical outcomes, but CCTA led to less ICA (adjusted HR 0.81; 95% CI [0.72–0.92]; p=0.001) without a change in coronary revascularization (HR 1.03; 95% CI [0.87–1.21]) or prescription of preventive therapy. This was at the expense of longer hospitalizations (median increase 0.21 days) and higher cost.30,31 These results are in contrast to prior trials that have demonstrated a reduction in both the cost of hospitalization and length of stay with CCTA versus usual care in lower-risk patients.19
A follow-up analysis of the VERDICT trial evaluated the prognostic role of stenosis by CCTA.32 That study included 978 patients who underwent ICA and CCTA and followed them for a median of 4.2 years for a composite of all-cause death, non-fatal recurrent MI, hospital admission for refractory myocardial ischemia, or heart failure. All CCTAs were blinded to clinical care, and ≥50% stenosis was defined as obstructive. Overall, the association between ≥50% stenosis and outcomes was similar between CCTA (HR 1.74; 95% CI [1.22–2.49]; p=0.002) and ICA (HR 1.54; 95% CI [1.13–2.11]; p=0.007). High-risk features, defined as obstructive left main or proximal left anterior descending artery stenosis and/or multivessel disease, were similarly associated with worse outcomes on both CCTA (HR 1.56; 95% CI [1.18–2.07]; p=0.002) and ICA (HR 1.28; 95% CI [0.98–1.69]; p=0.07). These data suggest that luminal stenosis by CCTA is an important prognostic sign for poor outcomes in those presenting with NSTEMI.
The ability of CCTA to visualize plaque and identify high-risk plaque features may aid in the classification of MI (e.g. type I versus II) and the identification of culprit lesions in patients with multivessel disease. Small studies of CCTA in patients with ACS have shown that different plaque characteristics can identify culprit plaque. For example, remodeling index (RI) has been shown to be associated with culprit lesions in patients with ACS, with an RI ≥1.23 associated with a 12-fold increased odds of culprit lesion (OR 12.3; 95% CI [2.9–68.7]; p<0.01).33 Similarly, Hoffman, et al. showed that beyond luminal stenosis, remodeling index and plaque area were higher in culprit versus non-culprit lesions in patients with ACS.34
Taken together, these studies demonstrate the excellent accuracy of CCTA in detecting obstructive CAD in patients with NSTEMI and illustrate the safety of this diagnostic modality in this patient population. In the era of the COVID-19 pandemic, the diagnostic accuracy of CCTA has been leveraged to safely evaluate patients with COVID-19 and elevated cardiac biomarkers.35 To accomplish this, many sites have increased the availability of CCTA to off-hours (5 pm–11 pm).35 CCTA also has the benefit of reducing staff contact time with patients to reduce the risk of COVID-19 transmission, leading to increases in use during the pandemic.35 In general, although CCTA has demonstrated reductions in LOS and healthcare costs in comparison with standard care in lower-risk patient populations, the evidence for similar reductions in high-risk patients is not currently present. Further studies are needed to clarify the role of CCTA in patients with NSTEMI.
Limited data exist on the role of FFRCT in patients with established ACS. In a study of 48 patients with ACS and deferred intervention on non-culprit lesions, non-culprit lesion FFRCT ≤ 0.8 was associated with increased major adverse cardiac events over 19.5 months (adjusted HR 1.56; 95% CI [1.01–2.83]; p=0.048).36 An ongoing trial is evaluating the ability of FFRCT to triage patients with high-risk ACS in the ER in comparison with invasive FFR.24 The role of FFRCT in the evaluation of non-culprit lesions after STEMI was investigated in a single-center prospective study that evaluated 60 patients with recent STEMI who underwent CCTA and invasive FFR 1 month after STEMI. Compared with >50% luminal stenosis by CCTA, FFRCT improved the accuracy (72% versus 64%, p=0.033) and specificity (66% versus 49%, p < 0.001) but not sensitivity (83% versus 93%, p=0.15) to predict invasive FFR ≤ 0.8. The performance in that study population was generally lower than what was observed in stable disease in the NXT trial (sensitivity 86% and specificity of 79%), which may be related to smaller vessel volume. These findings suggest a limited application of FFRCT after STEMI, and further refinement of the computational model may be needed in this population.
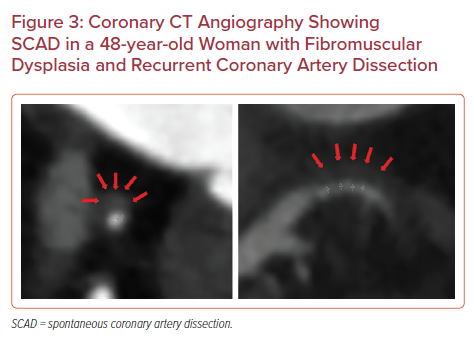
Although acute plaque rupture and resulting type I MI is often the immediate concern in NSTEMI, alternative pathologies (e.g. spontaneous coronary artery dissection [SCAD], coronary embolism and vasospastic disease) are becoming increasingly recognized as of importance. SCAD often occurs in relatively young women and disproportionately in the peripartum period (up to 18% of women with SCAD). The vast majority of patients with SCAD are managed conservatively owing to the risk of propagating the dissections with coronary intervention. Therefore, non-invasive imaging is often required to identify the location and extent of SCAD to guide therapy (Figure 3).37
Atheroma Phenotyping with CCTA
Coronary atherosclerosis is often graded based on luminal stenosis. CCTA enables plaque characterization, which is being increasingly recognized as an important predictor of clinical outcome.15 Compared with invasive modalities (e.g. intracoronary imaging), CCTA allows a full coverage of the coronary tree and is not limited by the depth of imaging, although it has lower spatial resolution (~5 mm with CCTA compared with 10 μm for optical coherence tomography [OCT] and 100 μm for intravascular ultrasound [IVUS]). Plaques can be characterized by the presence or absence of calcification, overall plaque volume, and high-risk plaque features (low attenuation, napkin ring sign, macrocalcification, and positive remodeling).
In an analysis of 472 patients who underwent CCTA in the ROMICAT II trial (32 with ACS), CTA-specific thresholds for plaque burden and degree of stenosis performed significantly better than IVUS-derived thresholds (p < 0.05), with minimal luminal area also having a modestly superior performance (p=0.066).38 In the SCOT-HEART trial, patients with both obstructive disease and adverse plaque features (low attenuation or positive remodeling) had a 10-fold increase in coronary heart disease death or non-fatal MI compared with patients with normal coronary arteries (HR 11.50; 95% CI [3.39–39.04]; p<0.001).39
CCTA can also provide insights regarding pericoronary adipose tissue (PCAT), which also seems to have prognostic information, which may be due to local inflammation.40 Fat attenuation index (FAI) ≥−70.1 HU has been linked to a significant increase in cardiac mortality (adjusted HR 9.04; 95% CI [3.35–24.4]; p<0.0001) in a large analysis of 1,872 patients who underwent CCTA.41 Quantitative analysis of peri-coronary fat may also facilitate the identification of vulnerable plaques and vulnerable patients during ACS presentation.42,43
Role of CCTA for Evaluation of Coronary Stents, Bypass Grafts and for PCI Planning
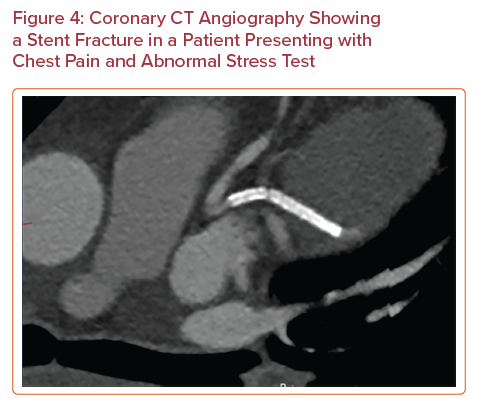
Advances in CT technology have improved spatial resolution and now enable evaluation of the patency of some coronary stents. A meta-analysis of 35 studies reporting on 4,131 stents showed that the pooled positive likelihood ratio was 14.0 and the pooled negative likelihood ratio was 0.10 for diagnosis of in-stent restenosis of ≥50%.44 The 2021 SCCT expert consensus document on CCTA concluded that it is appropriate to use CCTA to evaluate stents (≥3.0 mm in diameter) using special imaging protocols for image optimization, and it may be appropriate to image smaller stents (<3.0 mm in diameter) with known thin struts (<100 µm) in proximal vessels.45 The data surrounding the use of CCTA for the identification of in-stent thrombosis are limited. In-stent thrombosis is more conventionally diagnosed via ICA, IVUS and OCT. A 2011 study compared the performance of 64-slice CCTA and OCT for diagnosis of stent thrombosis in 79 patients who presented at a mean interval of 24 hours after the onset of acute chest pain within a month of the initial coronary event.46 CCTA was found to have a sensitivity of 95%, specificity of 93%, PPV of 83% and NPV of 98%. Although these data are limited to a small population of patients, the study serves as a promising proof of concept for the use of CCTA in this population. CCTA also enables evaluation of stent morphology and identification of the stent fracture non-invasively (Figure 4).
CCTA allows fairly accurate evaluation of CABG. Because grafts are typically larger than and subject to less motion artifact than native coronary arteries, CCTA is particularly useful in this population.45 A 2016 meta-analysis of 12 studies using a 64-detector CCTA protocol involving 959 patients demonstrated the high sensitivity (98%), specificity (98%) and discrimination (area under the receiver operating characteristic curve 0.99) of stenosis >50%, compared with ICA, regardless of conduit type (venous versus arterial).47 Evaluation of native coronary stenosis remains challenging in patients with prior CABG due to advanced atherosclerosis, often with diffuse calcification limiting luminal stenosis. Thus, CCTA for the evaluation of CABG patency may be most helpful in patients with known occluded native coronaries.
CCTA may also be helpful in procedure planning in patients with chronic total occlusions (CTOs) undergoing PCI. CCTA has the ability to demonstrate the course of the distal segment of occluded and large collateral vessels, which can offer a roadmap for interventions and may reduce procedure time. CCTA can additionally identify various coronary features that may be predictive of revascularization success and long-term outcomes, and identify myocardial coronary territory.48
Conclusion
CCTA is a versatile imaging modality that can play an appropriate role for coronary artery evaluation in several clinical settings. In the stable chest pain patient, it offers superior sensitivity to other non-invasive functional imaging modalities, leading to higher rates of statin and aspirin prescription rates. In low- to intermediate-risk patients with acute chest pain, it is not only safe, but potentially allows for reduced costs and LOS, and the diagnosis of non-coronary causes of acute, life-threatening chest pain that may otherwise be missed. The role of CCTA in higher-risk patients (such as those with confirmed NSTEMI) is evolving and it may provide a safe means of identifying patients without obstructive disease, for whom medical therapy may be sufficient. CCTA can also aid in plaque characterization, which can facilitate personalized therapeutic interventions and distinguish culprit from non-culprit lesions, or even non-atheromatous causes of ACS. CCTA also provides novel prognostic information with its ability to non-invasively derive information regarding plaque morphology and PCAT. Finally, it can also play an important role in evaluating prior coronary stents and CABG, and in the planning for PCI. The world of CCTA continues to expand with the development of new technologies (such as FFRCT), which will enhance the diagnostic abilities of CCTA and improve its specificity to identify hemodynamically significant stenoses.










