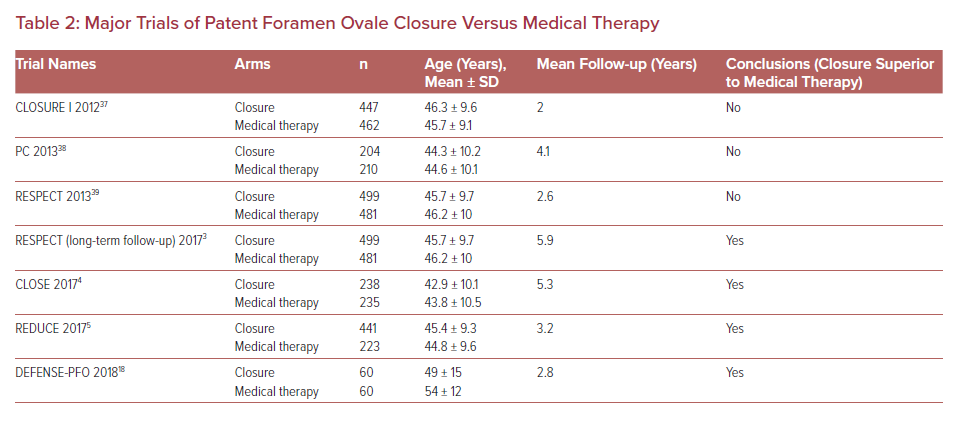
Cryptogenic stroke is defined as a stroke without definitive etiology, not attributed to cerebral atherosclerotic disease, cardiac arrhythmia or structural cardiac abnormalities. Patent foramen ovale (PFO) has been shown to be a route by which paradoxical embolus can cause strokes. While PFO is found in approximately 25% of the general population, PFOs are significantly more common among patients with cryptogenic stroke, with a prevalence of up to 40%.1,2 Historically, closure of PFO to reduce the risk of stroke due to paradoxical embolism has been controversial with conflicting data and conflicting expert opinion. However, in the last 3–4 years, several large randomized trials have solidified PFO closure as an option for young patients with cryptogenic stroke and a PFO.3–5 The RESPECT-LT trial, which extended follow-up to a median of 5.9 years, linked PFO closure to a significant 45% reduction in risk for stroke compared with medical therapy alone. This pivotal trial led to the Food and Drug Administration (FDA) approval of the Amplatzer PFO Occluder (Abbott) device in the US. The mean age in RESPECT-LT was 46 years, with patients >60 years excluded from enrollment.3 Similarly, the mean age in the CLOSE and REDUCE trials was 43 and 45 years, respectively.4–6
Lack of data in older patients has hindered the development of clinical guidelines for PFO closure. In general, older patients are at higher risk for stroke based on vascular risk factors alone, and the presence of a PFO is often an incidental finding. Conversely, older patients may also have additional risk factors that predispose to paradoxical embolism, such as acquired hypercoagulable states and immobility leading to venous thromboembolism. Younger patients with traditionally fewer vascular risk factors have a higher probability of a true cryptogenic stroke, and a longer lifetime risk of recurrent stroke. This fact, in combination with the data from the three main randomized controlled trials – RESPECT-LT, CLOSE and REDUCE and the several meta-analyses that followed – has shifted societal guidelines towards recommending PFO closure in younger patients with cryptogenic stroke.7–12 This raises questions. What is the definition of young? Is there an age cut off beyond which PFO closure should not be offered? Is there additional evaluation that should be considered in older patients before considering closure? As the population ages and PFOs are frequently discovered during cardiac evaluations, it is not surprising that older patients with more comorbidities are being referred to cardiologists for evaluation and consideration of PFO closure. This review will explore the history of PFO closure, emphasizing data in older adults. We propose a practical algorithm for evaluation and treatment of older adults with cryptogenic stroke and PFO while we await additional trials in this important population.
History of Patent Foramen Ovale Closure
The foramen ovale is a cardiac shunt present in the embryologic phase to bypass the lungs. This passageway normally closes at birth, once the lungs are functional and the left atrial pressure surpasses the right atrial pressure.13 However, this connection between the atria persists in approximately one in four adults.14 In general, most patients with PFO remain asymptomatic throughout their lifetime. However, it has been demonstrated that PFO is associated with paradoxical embolism and can lead to cryptogenic stroke and arterial emboli when a right to left shunt is present either transiently or permanently.1 PFO closure to protect against paradoxical emboli was first proposed in 1992.15 Multiple devices have been designed for the percutaneous closure of PFOs. Nevertheless, until recently it was unclear whether PFO closure offers any benefits compared to medical therapy.
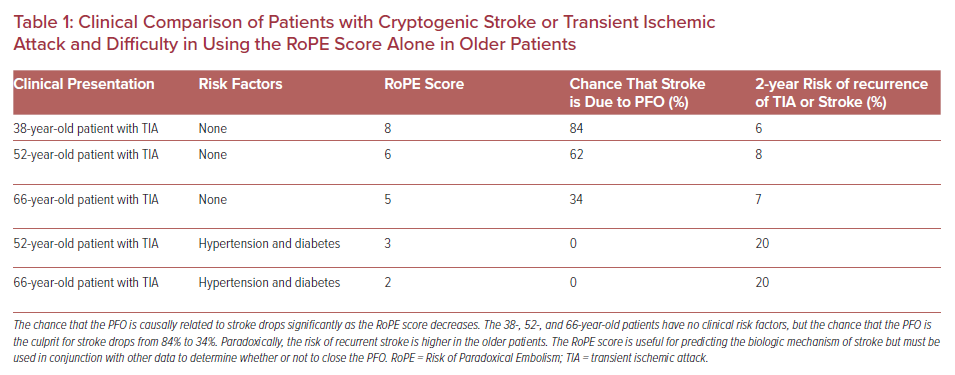
In 2013, the RoPE study proposed a scoring method to stratify the likelihood of the index stroke being PFO-related as opposed to the PFO being a bystander finding.16 The RoPE score uses just six clinical variables (hypertension, diabetes, smoking status, prior history of cerebral ischemia, imaging evidence of cortical infarct, and age) to predict the likelihood that the stroke is due to PFO as well as risk of recurrent stroke/transient ischaemic attack (TIA). Younger patients receive more points in the RoPE score, with additional points given for the clinical variables. A high RoPE score suggests the PFO is more likely to be the cause of stroke. While this is helpful in categorizing strokes as likely cryptogenic versus PFO-related, caution is warranted when using the score. The RoPE score does not provide a cut-off value for recommending PFO closure, nor does it provide guidance on whether to treat patients with PFO closure or medical therapy. It also does not include some nuanced variables that might sway decision-making on an individual basis such as anatomical considerations (long tunnel, large shunt, hypermobile septum) or specific AF risk factors (heart failure, left atrial enlargement). While it is a helpful tool it cannot be used by itself to decide treatment. Table 1 outlines several clinical scenarios and associated RoPE scores to illustrate the complexity of the RoPE score in older patients.
Before 2016, randomized controlled trials produced contradictory data and lacked long-term follow-up. However, the results of three important randomized controlled trials published in 2017 changed the direction of PFO management. RESPECT-LT, CLOSE and REDUCE included follow-up of 3.2–5.9 years and all three trials showed significant reduction in risk of recurrent stroke in the closure groups when compared with medical therapy (Table 2).3–6 The RESPECT-LT trial led to the FDA approval of the Amplatzer PFO Occluder device in the US for patients between the ages of 18–60 years with a PFO and cryptogenic stroke. Subsequently the FDA has approved closure with the Gore Cardioform Septal Occluder (Gore). In our experience, since 2016 the number of referrals for PFO closure has dramatically increased, including patients over 60 years of age.
Patent Foramen Ovale and Cryptogenic Stroke in Patients Aged >60 Years
The prevalence of PFO is almost three times higher in elderly patients with cryptogenic strokes compared with patients of the same age with a known cause of stroke, suggesting that elderly patients may derive benefit from PFO closure.17 Although the RESPECT-LT, CLOSE and REDUCE trials established the superiority of PFO closure versus medical therapy for preventing recurrent ischemic stroke, none of them included patients older than 60 years. While a randomized controlled trial comparing patients aged >60 years with PFO closure versus medical therapy has not been performed, a few recent small studies suggest that PFO closure in the elderly is safe and may decrease the risk of recurrent stroke. The DEFENSE-PFO trial included patients older than 55 years and demonstrated positive results for PFO closure compared with medical therapy alone, although the mean age was only 51.8 years and total enrollment only 120 patients with 2 years follow-up. The DEFENSE-PFO results support PFO closure over medical therapy in middle-aged patients with high-risk anatomic features including PFO size, atrial septal hypermobility, and atrial septal aneurysm.18
It is unclear to what extent elderly patients derive benefit from PFO closure. Small retrospective studies have not provided definitive answers and – similar to DEFENSE-PFO – they have limited follow-up duration and number of enrollees aged >55, with even fewer enrollees aged >65. For example, one single-center retrospective cohort study identified 14 elderly patients (mean age 75.2 years) with a high-risk PFO and prior cerebrovascular event who underwent PFO closure. After following up for 2.6 ± 1.8 years, none of them experienced recurrent cerebrovascular events and the complications were not higher than expected.19 While this was a positive result in this very advanced age group, the small sample size and short follow-up duration are limitations. Another contemporary series with a longer mean follow-up period (4.5 years) included 458 patients who underwent PFO closure for cryptogenic cerebral ischemia.20 The 151 patients who were older than 55 years had a higher risk of recurrent ischemia, with age being the only independent predictor. Most events were more than 3 years out from the procedure and therefore not associated with peri-procedural complications. Neither group had significant residual shunting that would account for recurrent events being higher due to PFO-related mechanisms, and complexity of the PFO anatomy was not different between the two groups. The authors concluded that recurrent events were most likely associated with underlying conditions in the older population as might be predicted from the RoPE score.
Proponents of PFO closure point out that the procedure is safe and low risk, but without randomized clinical trial data in older patients we can only weigh up the clinical variables, vascular risk factors, lifestyle and life expectancy and make an appropriate judgement for each individual patient.
Evaluation and Treatment Considerations
Early guidelines for the closure of PFO after stroke were written before 2017, prior to publication of REDUCE, CLOSE and DEFENSE-PFO trials that demonstrated positive results in favor of PFO closure.21,22 However, two newer guidelines have been released: a position paper from the European Heart Journal in 2018 and an advisory update from the American Academy of Neurology in 2020.23,24 There are some differences between these guidelines. Nevertheless, both concur that PFO closure should be considered in elderly patients with cryptogenic stroke and no evident alternative mechanism other than paradoxical embolization. Neither guideline provides a specific age cut off or a combination of risk factors that would preclude PFO closure or guide further workup. The following sections outline important considerations for the cardiologist when evaluating older patients with possible PFO-related stroke.
Initial Cardiac Evaluation
PFO with a right to left shunt can be detected by using ultrasound with color Doppler imaging in combination with agitated saline contrast and maneuvers like Valsalva that transiently increase right atrial pressure. The American Society of Echocardiography defines a positive bubble study as the appearance of microbubbles in the left atrium within three to six cardiac beats of opacification of the right atrium either at baseline or by using the Valsalva maneuver to promote right to left shunting.25 Transthoracic echocardiography (TTE) should be used as the first imaging modality at any age to rule out intra-cardiac shunting, including possible cardiac abnormalities that could be associated with an increased risk for paradoxical emboli (e.g. atrial septal aneurysm). In addition, a TTE provides information to rule out structural heart diseases such as left ventricular (LV) dysfunction or valvular heart disease that might predispose to left sided thrombus or an increased risk of AF. LV dysfunction – even in the absence of LV thrombus – in a patient aged >55 years, strongly suggests a non-PFO mechanism of stroke.26,27
Transesophageal echocardiography (TEE) has a higher sensitivity for and provides better visualization of the PFO, especially defining the direction of the flow through the shunt and distinguishing it from pulmonary arteriovenous shunting. Therefore, if the TTE is negative, we recommend performing a TEE if there is high suspicion of a PFO.28,29 Transcranial Doppler can detect right to left shunt by identifying bubbles from agitated contrast saline in arteries that supply perfusion to the brain. However, the limitation of this modality is that it does not provide anatomic characteristics or location of the shunt.30 Although less sensitive than TEE, other potential modalities that can detect a PFO are cardiovascular MRI and multi-detector CT.
TEE can also visualize the aorta and provide information on aortic atheroma. While this finding may confirm a diagnosis of vascular disease, it should not by itself preclude closure of a PFO and should be treated similarly to stable coronary artery or carotid artery disease.
Initial Neurologic Evaluation
Patients referred with cryptogenic stroke should have already had routine brain imaging with MRI as well as a cerebrovascular evaluation with magnetic resonance angiography (MRA) or CT angiography (CTA) or carotid duplex. Cortical defects or multiple areas of infarct point to an embolic source of stroke. Lacunar strokes may be caused by small emboli but in general are thought to be non-embolic and not an indication for PFO closure (Figure 1).31
Carotid duplex and MRA/CTA should also be first-line studies, as carotid artery dissection or atherosclerotic lesions are a common cause of TIA and embolic stroke. Carotid disease with evidence of ≥70% stenosis and an ipsilateral associated cortical imaging defect should prompt further evaluation by a vascular surgeon and neurologist rather than PFO closure. Stable carotid disease ≥70% should not in itself exclude PFO closure, but increases the likelihood that a stroke is unrelated to the PFO.
Monitoring for AF
Extended cardiac monitoring in patients at high risk for AF is recommended. European guidelines suggest that patients aged 55–65 years (level of evidence [LOE] C) or >65 years (LOE B) with a high risk for AF should be monitored for 6 months with an implantable cardiac monitor.24 The 2019 Focused Update of the American College of Cardiology/American Heart Association/Heart Rhythm Society 2014 Guidelines for the Management of Patients with AF recommend extended cardiac monitoring for any patient older than 40 years with suspected AF.32 The American Academy of Neurology recommends at least 28 days of cardiac monitoring in patients who are being considered for PFO closure (LOE B).23
The type and length of AF monitoring remain controversial. The European position paper on the management of patients with PFO suggests that implantable cardiac monitoring (ICM) is useful in patients over 65 and can be considered for those under 55 and at high risk for AF, where high risk features include uncontrolled hypertension, structural heart disease such as LV hypertrophy or left atrial enlargement, uncontrolled diabetes or congestive heart failure.24 Considering all of these documents, as well as the epidemiological burden of AF in people older than 55 years versus those aged 40–55, we propose an algorithm that includes the use of extended intracardiac monitoring for at least 6 months to rule out AF prior to the PFO closure in patients over the age of 55 years (Figure 1). After 6 months, these patients should have ICM continued for the full battery life of the monitor, given the possibility of a late recurrent paroxysmal AF. Importantly, the presence of short bursts of AF might not exclude the need for PFO closure, especially if the PFO has anatomical high-risk features.
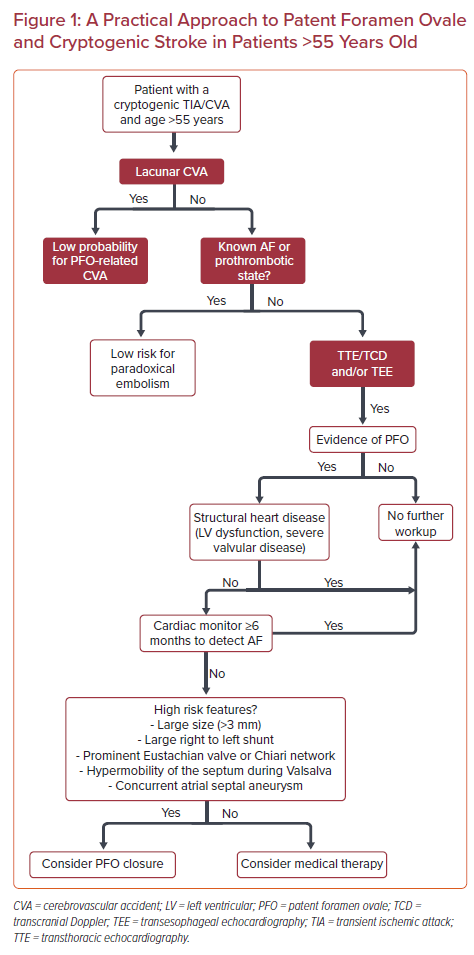
In addition to the more complex intracardiac monitoring, a routine 12-lead ECG should always be performed as part of the initial evaluation. ECG evidence of left atrial enlargement using the P-wave terminal force in lead V1 can be a marker of left atrial abnormality and has been associated with cardiogenic stroke.33 This finding might point to an atrial pathology rather than paradoxical embolism as the cause of the stroke. ECG findings data should be taken in conjunction with the associated imaging, clinical history and RoPE score.
Anatomical Considerations
Multiple studies have associated anatomical characteristics of PFOs with initial and recurrent paradoxical embolism and stroke. These include large PFO with an opening of >3 mm and large right to left shunt. An atrial septal aneurysm defined as atrial septal bowing ≥10 mm has been associated with a higher risk for cryptogenic strokes, although this finding was contradicted by the Patent Foramen Ovale in Cryptogenic Stroke Study, which demonstrated no association between the presence of PFO and atrial septal aneurysm.34 Large eustachian valves and/or Chiari networks may contribute to paradoxical embolism by diverting blood from the inferior vena cava to the interatrial septum.35 While the role of high-risk anatomic features in decision making is not clear, we suggest that these findings be used in tandem with other patient-specific risk factors and features to help determine the need for PFO closure.
Multidisciplinary Evaluation
All patients who are considered for PFO closure should be evaluated by a multi-disciplinary team, including a neurologist and a cardiologist, in particular the older patient with evidence of vascular disease. The input of a neuroimaging expert should be considered given that it is important to determine if ischemic strokes are more likely embolic or due to intrinsic vascular disease and to help interpret unusual findings in the cerebral vasculature. PFO-related strokes are often associated with different vascular territories. For example, patients with a PFO and a history of cryptogenic stroke more often have posterior circulation involvement, whereas patients with other comorbidities such as hypertension and left atrial cardiomyopathy had involvement of multiple vascular territories. Patients with carotid plaque and dyslipidemia more often had anterior vascular territory strokes.36
Risk factors for venous thromboembolism such as cancer or hypercoagulable disorders need to be considered and in cases of complex hypercoagulable disorders a hematologist should be part of the multidisciplinary team. Primary care physicians can provide input regarding patient specific factors (including frailty and life expectancy) that shape the risk-to-benefit ratio.
Conclusion
In an age of patient-centered care and with the available data, we should not exclude patients over 55 – or even over 65 years – from PFO closure in the setting of cryptogenic stroke. While a low RoPE score may suggest that the PFO is not the causal mechanism of stroke, a low RoPE score is not a contraindication to closure. PFO closure is a relatively low-risk procedure, and patient selection and shared decision making are critical. Careful multi-specialty evaluation is important, including long-term cardiac monitoring specifically looking for occult AF. A large size and high-risk morphology of the PFO may also influence a decision to perform closure. Trial data in older patients is limited and additional trials are needed to assess the benefits of PFO closure as our population ages and stroke remains a major cause of morbidity and mortality for people over the age of 60 years.